Global Extreme Lateral Interbody Fusion (XLIF) Surgery Market Size, Share - Forecast 2034

Extreme Lateral Interbody Fusion (XLIF) Surgery Market By End-User (Spinal Surgery Centers, Hospitals, Others), By Product Type (XLIF Interbody Fusion Systems, XLIF Interbody Cages), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1078.55 Million | USD 1720.38 Million | 4.78% | 2024 |
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Industry Perspective
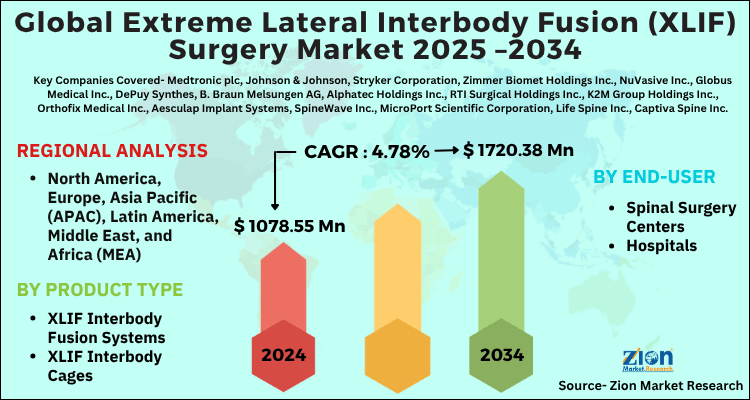
The global extreme lateral interbody fusion (XLIF) surgery market size was worth around USD 1078.55 Million in 2024 and is predicted to grow to around USD 1720.38 Million by 2034 with a compound annual growth rate (CAGR) of roughly 4.78% between 2025 and 2034. The report analyzes the global extreme lateral interbody fusion (XLIF) surgery market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the extreme lateral interbody fusion (XLIF) surgery industry.
The report delves deeper into several crucial aspects of the global extreme lateral interbody fusion (XLIF) surgery market. It includes a detailed discussion of existing growth factors and restraints. Future growth opportunities and challenges that impact the extreme lateral interbody fusion (XLIF) surgery industry are comprehensively addressed in the report.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Overview
Extreme lateral interbody fusion (XLIF) surgery is a surgical procedure that is used to treat several spinal conditions. It is a minimally invasive procedure and involves accessing the spine using a side or lateral approach. During the process, the affected area is fused which helps in stabilizing the spine and alleviating any associated pain. XLIF surgeries are conducted using specialized surgical equipment and the involved medical professionals are required to leverage the benefits of imaging tools. The surgeon makes a small incision on the patient’s side and gains access to the spine for removing damaged or degenerated discs between the vertebrae. The incision is typically made in the lower back or the flank region. Once the removal of degenerated discs is complete, the vertebrae are fused with the help of bone grafts.
Key Insights
- As per the analysis shared by our research analyst, the global extreme lateral interbody fusion (XLIF) surgery market is estimated to grow annually at a CAGR of around 4.78% over the forecast period (2025-2034).
- Regarding revenue, the global extreme lateral interbody fusion (XLIF) surgery market size was valued at around USD 1078.55 Million in 2024 and is projected to reach USD 1720.38 Million by 2034.
- The extreme lateral interbody fusion (XLIF) surgery market is projected to grow at a significant rate due to increasing demand for minimally invasive spinal procedures, advancements in surgical technologies, and the rising prevalence of degenerative spinal conditions among the aging population.
- Based on End-User, the Spinal Surgery Centers segment is expected to lead the global market.
- On the basis of Product Type, the XLIF Interbody Fusion Systems segment is growing at a high rate and will continue to dominate the global market.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Growth Drivers
Growing prevalence of spinal disorders to drive market growth
The global extreme lateral interbody fusion (XLIF) surgery market is projected to grow owing to the increasing prevalence of spinal disorders across the globe. Conditions such as degenerative disc disease, herniated discs, and spinal stenosis are becoming more prevalent driven by the sedentary lifestyle adopted by the general population, especially after Covid-19. A report by the Centers for Diseases Control and Prevention (CDC) claims that nearly 15% of the American population leads a sedentary lifestyle. Additionally, the World Health Organization (WHO) reports that every year, around 250,000 to 500,00 people worldwide suffer from some form of spinal cord injury (SPI). Alongside this, favorable outcome and positive response from the patient in terms of satisfaction has helped build good regulation for the procedure in the medical community. XlIF is a popular choice of treatment since this technique has a success rate of over 94% in most cases.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Restraints
High cost of XLIF surgery to restrict market expansion
The cost of XLIF surgery is higher when compared to other forms of medical processes used for spinal cord-related medical issues. In India, the cost may range between INR 2,50,000 to INR 5,00,000. For an emerging economy such as India, the expense of the treatment and post-treatment care may be difficult for a large portion of the lower income families.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Opportunities
Growing investment toward technological advancement to provide growth opportunities
The global extreme lateral interbody fusion (XLIF) surgery market is expected to come across more growth opportunities owing to the increasing investment toward technological advancements in the medical sector. In May 2022, Viseon Inc. announced that the company's latest offering, Viseon MaxView Imaging Platform, was successfully used in more than 1000 spine surgery cases in the US. MaxView is a highly advanced intraoperative imaging system that allows an unobstructed and highly magnified view of the surgical corridor.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Challenges
Existing competition from alternate methods of spine-related surgery to challenge market expansion
The extreme lateral interbody fusion surgery industry may register certain challenges against its growth trajectory due to the existing demand for alternate methods used in spine-related surgeries. Procedures such as posterior lumbar interbody fusion (PLIF), transforaminal lumbar interbody fusion (TLIF), and anterior lumbar interbody fusion (ALIF) are used extensively for spine stabilization. Over 30,500 lumbar fusion procedures are conducted in the US every year. For instance, in January 2023, orthopedic and neurosurgical spine teams from the Lenox Hill Hospital became the first surgeons in New York to use the Q Guidance System by Stryker for spinal navigation and guidance in surgery. The technology leverages X-ray imaging to construct a 3-D and real-time model of the spine which helps doctors deliver better during surgical procedures. It also already received Food & Drugs Administration (FDA) approval to be used with pediatric patients.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Segmentation
The global extreme lateral interbody fusion (XLIF) surgery market is segmented based on end-user, product type, and region.
Based on End-User, the global market segments are spinal surgery centers, hospitals, and others. The highest growth was observed in the hospitals segment in 2022 especially centers with dedicated spine centers or departments. Most of the large hospitals are equipped with doctors, medical professionals, and diagnostic tools required to conduct every form of surgery. For instance, in March 2023, the spinal surgery centers are likely to witness higher growth due to growing efforts toward increasing investments. The regional governments along with private companies are working toward increasing investment and funding in such centers. Companion Spine proudly announced the addition of USD 5 million in funding bringing its Series A financing to a total of USD 60.1 million. The company has already received USD 55 million by February 2022. It is a spine pain-management company that mainly deals with the treatment of degenerative disc diseases (DDD) and lumbar spinal stenosis (LSS).
Based on Product Type, the extreme lateral interbody fusion surgery industry segments are XLIF interbody fusion systems and XLIF interbody cages. The demand for both product types is equally high. The former product type is a comprehensive set of instruments, implants, and accessories required for the XLIF surgical procedure. It includes specialized retractors, dilators, bone graft instruments, screws, rods, and other instruments. XLIF interbody cages, on the other hand, are implantable devices specifically designed for interbody fusion in XLIF surgeries. They are placed between adjacent vertebrae to restore disc height, decompress nerves, and facilitate fusion. These cages are considered the primary component of the procedure and hence are more commonly used. In 2018, NuVasive, Inc, a leading player in the market, introduced the XLIF® Lordotic Expandable (XLX) Interbody System after receiving 510(K) approval from the US FDA.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Extreme Lateral Interbody Fusion (XLIF) Surgery Market |
| Market Size in 2024 | USD 1078.55 Million |
| Market Forecast in 2034 | USD 1720.38 Million |
| Growth Rate | CAGR of 4.78% |
| Number of Pages | 206 |
| Key Companies Covered | Medtronic plc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet Holdings Inc., NuVasive Inc., Globus Medical Inc., DePuy Synthes, B. Braun Melsungen AG, Alphatec Holdings Inc., RTI Surgical Holdings Inc., K2M Group Holdings Inc., Orthofix Medical Inc., Aesculap Implant Systems, SpineWave Inc., MicroPort Scientific Corporation, Life Spine Inc., Captiva Spine Inc., CoreLink Surgical LLC, CTL Medical Corporation, Providence Medical Technology Inc., Safe Orthopaedics, Spinal Elements Holdings Inc., Spineology Inc., Wenzel Spine Inc., Xenco Medical., and others. |
| Segments Covered | By End-User, By Product Type, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 - 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Regional Analysis
North America to hold control over the highest market share
The global extreme lateral interbody fusion (XLIF) surgery market will witness the highest growth in North America during the forecast period. This is mainly due to the presence of a robust medical infrastructure in the US area along with greater access to primary and advanced medical care in the Canadian region. As per reports from The Commonwealth Fund, nearly 37% of the Canadian population was covered by some form of medical insurance between 2017 and 2018. Moreover, the US has registered a high rate of medical tourism.
The United States International Trade Commission estimates that the US is visited by around 100,000 to 200,000 people annually for medical reasons. Europe is another significant market for XLIF surgeries with countries such as Germany, the United Kingdom, France, and Italy showing a well-developed healthcare system and the increasing prevalence of spinal conditions. The Council of Europe estimates that around 11000 new cases of spinal-related conditions are added every year to the existing list of patients with these issues.
Extreme Lateral Interbody Fusion (XLIF) Surgery Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the extreme lateral interbody fusion (XLIF) surgery market on a global and regional basis.
The global extreme lateral interbody fusion (XLIF) surgery market is dominated by players like:
- Medtronic plc
- Johnson & Johnson
- Stryker Corporation
- Zimmer Biomet Holdings Inc.
- NuVasive Inc.
- Globus Medical Inc.
- DePuy Synthes
- B. Braun Melsungen AG
- Alphatec Holdings Inc.
- RTI Surgical Holdings Inc.
- K2M Group Holdings Inc.
- Orthofix Medical Inc.
- Aesculap Implant Systems
- SpineWave Inc.
- MicroPort Scientific Corporation
- Life Spine Inc.
- Captiva Spine Inc.
- CoreLink Surgical LLC
- CTL Medical Corporation
- Providence Medical Technology Inc.
- Safe Orthopaedics
- Spinal Elements Holdings Inc.
- Spineology Inc.
- Wenzel Spine Inc.
- Xenco Medical.
The global extreme lateral interbody fusion (XLIF) surgery market is segmented as follows;
By End-User
- Spinal Surgery Centers
- Hospitals
- Others
By Product Type
- XLIF Interbody Fusion Systems
- XLIF Interbody Cages
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
Extreme lateral interbody fusion (XLIF) surgery is a surgical procedure that is used to treat several spinal conditions.
The global extreme lateral interbody fusion (XLIF) surgery market is expected to grow due to rising prevalence of spinal disorders, increasing demand for minimally invasive surgical procedures offering faster recovery and less pain, advancements in surgical technologies.
According to a study, the global extreme lateral interbody fusion (XLIF) surgery market size was worth around USD 1078.55 Million in 2024 and is expected to reach USD 1720.38 Million by 2034.
The global extreme lateral interbody fusion (XLIF) surgery market is expected to grow at a CAGR of 4.78% during the forecast period.
North America is expected to dominate the extreme lateral interbody fusion (XLIF) surgery market over the forecast period.
Leading players in the global extreme lateral interbody fusion (XLIF) surgery market include Medtronic plc, Johnson & Johnson, Stryker Corporation, Zimmer Biomet Holdings Inc., NuVasive Inc., Globus Medical Inc., DePuy Synthes, B. Braun Melsungen AG, Alphatec Holdings Inc., RTI Surgical Holdings Inc., K2M Group Holdings Inc., Orthofix Medical Inc., Aesculap Implant Systems, SpineWave Inc., MicroPort Scientific Corporation, Life Spine Inc., Captiva Spine Inc., CoreLink Surgical LLC, CTL Medical Corporation, Providence Medical Technology Inc., Safe Orthopaedics, Spinal Elements Holdings Inc., Spineology Inc., Wenzel Spine Inc., Xenco Medical., among others.
The report explores crucial aspects of the extreme lateral interbody fusion (XLIF) surgery market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed

-surgery-market-size.png)