Spine Surgery Devices Market Share, Size, Analysis and Forecast 2032

Spine Surgery Devices Market By Technology Type (Fusion Non-Fusion, Vertebral Compression Fracture, Spine Biologics, And Spinal Decompression) For Device Type Like Fusion Devices, Fracture Repair, Non-Fusion Devices And Arthroplasty- Asia Pacific Industry Perspective, Comprehensive Analysis, Size, Share, Growth, Segment, Trends, And Forecast, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
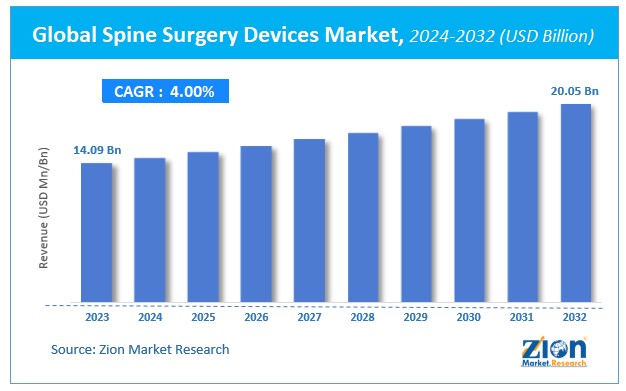
| USD 14.09 Billion | USD 20.05 Billion | 4% | 2023 |
Spine Surgery Devices Market Insights
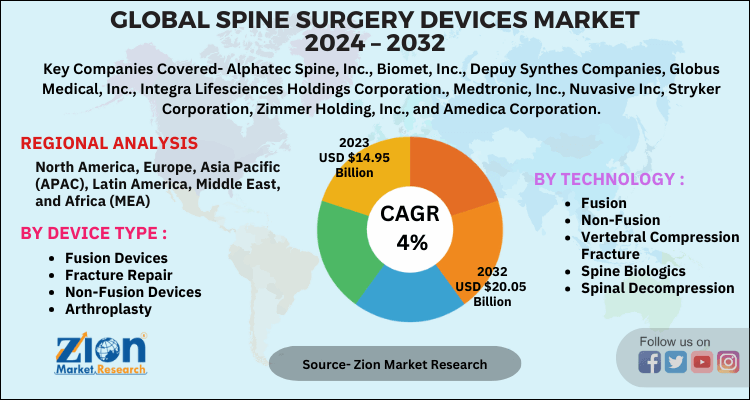
According to Zion Market Research, the global Spine Surgery Devices Market was worth USD 14.09 Billion in 2023. The market is forecast to reach USD 20.05 Billion by 2032, growing at a compound annual growth rate (CAGR) of 4% during the forecast period 2024-2032.
The report offers a comprehensive analysis of the market, highlighting the factors that will determine growth, potential challenges, and opportunities that could emerge in the Spine Surgery Devices Market industry over the next decade.
Spine Surgery Devices Market: Overview
The growth of the spine surgery market is propelled by the increasing number of geriatric and obese populations. The geriatric and obese populations are more susceptible to spine problems. Moreover, emerging technologies for minimally invasive spine surgery procedures and the increasing prevalence of spinal injuries are projected to fuel the expansion of the spine surgery market. Additionally, car and automobile accidents are the most reason for spine injuries, per annum, owing to car and automobile accidents are accounting for quite 35% of spine injuries and this may boost the expansion of the spine surgery device market.
On another hand, uncertainty in reimbursement, the high cost of surgery, and the paucity of education associated with spine treatment may hamper the expansion of the spine surgery market. Nonetheless, increasing demand for medical tourism in emerging markets is probably going to open new opportunities within the forecast period.
The spine is a made up of 26 discs known as the vertebrate. These vertebrate helps in protecting the spinal cord and allow us to stand and bend. Spine injuries are very serious because spinal cord contains the nerves that carry the message to body from brain and damage in spinal cord causes its function these may translate into the loss of muscle function and sensation. For the treatment of spinal surgery injuries number of technology are used like fusion non-fusion, vertebral compression fracture, spine biologics and spinal decompression. According to World Health Organization, every year 2.5 to 5.0 lakh people suffer from spine injuries and these injuries are mainly due to road traffic accidents and violence.
Spine Surgery Devices Market: Growth Factors
The key factors propelling the growth of this market are the increasing adoption rate of minimally invasive spinal surgeries, increasing incidences of obesity and degenerative spinal conditions & increasing technological advancements in spinal surgery. The spine is composed of 26 vertebrates stacked on top of one other and spinal column allowing us to stand upright twist & bend and provides the main support for our body. Thus, damage to the spinal cord causes a serious problem to the spinal column either temporarily or permanently. Patients facing issues opt for surgery as these days number of technologies are available including fusion, non-fusion, vertebral compression fracture, spine biologics, and spinal decompression. New product launches and technological advancements are anticipated to possess a positive influence on regional demand.
Increasing road accidents and violence are projected to boost the growth of spine surgery devices market. Rising prevalence of obesity is the main factor responsible for the growth of spine surgery devices market and this prevalence of obesity is increasing with changing lifestyle, lack of proper diet & exercise and increasing consumption of fast food. Increasing prevalence of spinal disorders is also growing the market of spine surgery device. However, lack of understanding of spine surgery reimbursement may hamper the growth of spine surgery devices market.
Spine Surgery Devices Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Spine Surgery Devices Market |
| Market Size in 2023 | USD 14.09 Billion |
| Market Forecast in 2032 | USD 20.05 Billion |
| Growth Rate | CAGR of 4% |
| Number of Pages | 180 |
| Key Companies Covered | Alphatec Spine, Inc., Biomet, Inc., Depuy Synthes Companies, Globus Medical, Inc., Integra Lifesciences Holdings Corporation., Medtronic, Inc., Nuvasive Inc, Stryker Corporation, Zimmer Holding, Inc., and Amedica Corporation |
| Segments Covered | By Device Type, By Technology And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Technology Segment Analysis Preview
Based on technology type spine surgery market has been segmented into fusion, non-fusion, vertebral compression fracture, spine biologics, and spinal decompression. In 2018, the fusion technology segment dominated the market of spine surgery with a 42% share of the market and but in the forecast, it is expected to slow down the growth rate due to emerging technologies in the spine biologics segment. In the forecast period, spine biologics technology is expected to come up as the leading segment with the highest growth rate.
Device Type Segment Analysis Preview
Based on device type, the market of spine surgery has been segmented into fusion devices, fracture repair, non-fusion devices, and arthroplasty. In 2018, the fusion device segment dominated the spine surgery market with a 45% market share and during the forecast period, the non-fusion market is anticipated to grow at a healthy rate due to increasing demand for minimally invasive surgery procedures.
Spine Surgery Devices Market: Regional Analysis
The Asia Pacific is projected to grow at the fastest growth shortly and this is due to increasing awareness and government support towards the industry. Based on the country, the market has been segmented as China, Japan, India, and the Rest of Asia-Pacific. In 2017, China was dominating the spine surgery device market followed by Japan while India held the 20% share of the Asia Pacific spine surgery device market. In the forecast period, China is expected to grow at a CAGR of 8% due to the increasing geriatric and obese population.
Key Market Players & Competitive Landscape
Key participants operating in the Asia Pacific spine surgery devices market include -
- Alphatec Spine
- Biomet
- Depuy Synthes Companies
- Globus Medical
- Integra Lifesciences Holdings Corporation.
- Medtronic
- Nuvasive Inc
- Stryker Corporation
- Zimmer Holding
- Amedica Corporation.
The Asia Pacific Spine Surgery Devices Market is segmented as follows:
By Device Type
- Fusion Devices
- Fracture Repair
- Non-Fusion Devices
- Arthroplasty
By Technology
- Fusion
- Non-Fusion
- Vertebral Compression Fracture
- Spine Biologics
- Spinal Decompression
By Region
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Table Of Content
Methodology
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed