Pharmaceutical Stability And Storage Services Market Size, Share & Forecast 2034

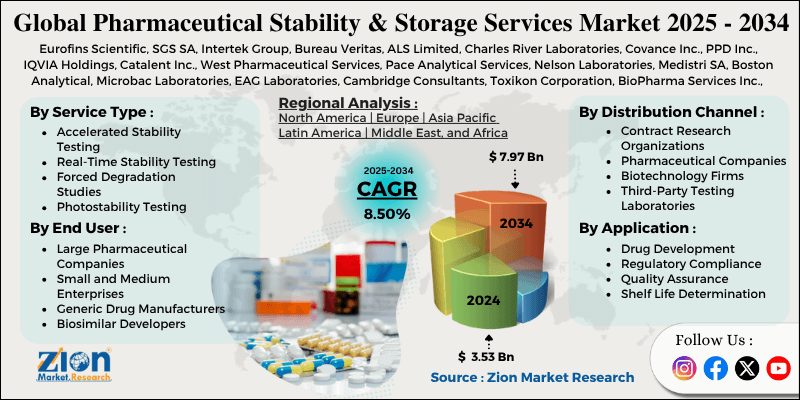
Pharmaceutical Stability And Storage Services Market By Service Type (Accelerated Stability Testing, Real-Time Stability Testing, Forced Degradation Studies, and Photostability Testing), By Application (Drug Development, Regulatory Compliance, Quality Assurance, and Shelf Life Determination), By Distribution Channel (Contract Research Organizations, Pharmaceutical Companies, Biotechnology Firms, and Third-Party Testing Laboratories), By End-User (Large Pharmaceutical Companies, Small and Medium Enterprises, Generic Drug Manufacturers, and Biosimilar Developers), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 3.53 Billion | USD 7.97 Billion | 8.50% | 2024 |
Pharmaceutical Stability And Storage Services Industry Perspective:
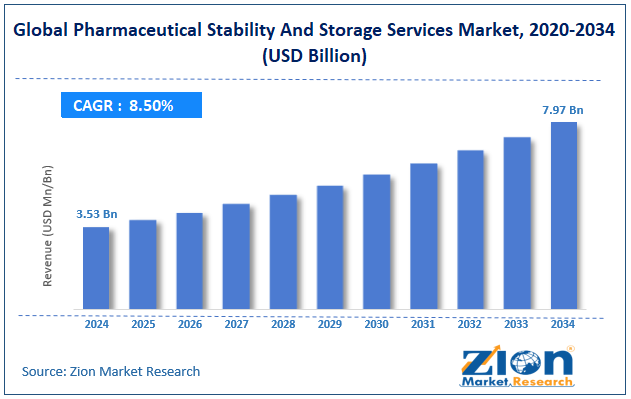
The global pharmaceutical stability and storage services market was valued at approximately USD 3.53 billion in 2024 and is expected to reach around USD 7.97 billion by 2034, growing at a compound annual growth rate (CAGR) of roughly 8.50% between 2025 and 2034.
Pharmaceutical Stability And Storage Services Market: Overview
Pharmaceutical stability and storage services involve specialized testing to check how well products maintain their quality, safety, and effectiveness over time in different environmental conditions. These services include comprehensive testing, such as temperature checks, humidity control, light exposure testing, and chemical breakdown analysis. Modern stability testing offers detailed records for regulatory approvals, shelf life proof, and storage condition advice during the product’s lifecycle. The market serves companies making new drugs, biosimilars, generic medicines, and medical devices that need stability data for approval. As drug formulas become more complex and regulations become stricter, an increasing number of companies are utilizing expert stability testing services.
The expanding pharmaceutical pipeline and stringent regulatory standards are expected to drive substantial growth in the pharmaceutical stability and storage services market over the forecast period.
Key Insights:
- As per the analysis shared by our research analyst, the global pharmaceutical stability and storage services market is estimated to grow annually at a CAGR of around 8.50% over the forecast period (2025-2034)
- In terms of revenue, the global pharmaceutical stability and storage services market size was valued at around USD 3.53 billion in 2024 and is projected to reach USD 7.97 billion by 2034.
- The pharmaceutical stability and storage services market is projected to grow significantly due to the increasing drug development activities, rising regulatory compliance requirements, and growing outsourcing trends in pharmaceutical testing.
- Based on service type, accelerated stability testing leads the segment and is expected to continue dominating the global market.
- Based on the application, regulatory compliance is expected to lead the market.
- Based on the distribution channel, contract research organizations are anticipated to command the largest market share.
- Based on end-users, large pharmaceutical companies are expected to lead the market during the forecast period.
- Based on region, North America is projected to lead the global market during the forecast period.
Pharmaceutical Stability And Storage Services Market: Growth Drivers
Increasing pharmaceutical research and development activities
The pharmaceutical stability and storage services market is growing as global pharmaceutical research and development investments rise across all therapy areas. Modern drug pipelines are becoming more complex with new formulations, combination products, and advanced delivery systems that need specialized stability testing.
Drug manufacturers are developing more complex molecules that necessitate comprehensive stability studies to understand their breakdown mechanisms and the optimal storage conditions required for their use. Biotechnology firms are developing new biologics and biosimilars that need unique stability tests because of their sensitive and complex structures. Generic drug companies are growing their product range and need stability data to prove bioequivalence and meet regulatory needs.
Stringent regulatory requirements and compliance standards
The pharmaceutical stability and storage services market is growing as health authorities worldwide introduce stricter rules for drug approval and market entry. Regulators now need full stability data that proves product quality, safety, and performance under different storage conditions throughout its shelf life.
Global guidelines are standardizing stability testing rules across regions, resulting in a steady demand for specialized testing. Good Manufacturing Practice (GMP) standards need clear documentation and validation of all stability tests to protect product quality and patient safety. The use of Quality by Design in stability testing requires more advanced analysis and better data interpretation.
Pharmaceutical Stability And Storage Services Market: Restraints
High costs and lengthy testing timelines
Despite rising demand, the pharmaceutical stability and storage services market faces challenges due to the large financial investment and long timelines needed for full stability testing. Long-term studies can last several years and require constant monitoring, which is a big burden for pharmaceutical firms.
Stability testing also needs specialized equipment, controlled storage rooms, and trained staff, all of which add to operating costs. Complex testing methods and advanced instruments require major capital and ongoing maintenance, which can be hard for some companies to afford. Regulatory updates may necessitate additional testing or modifications to the protocol, resulting in increased costs and potential delays.
Pharmaceutical Stability And Storage Services Market: Opportunities
Expanding outsourcing trends and specialized service demand
The pharmaceutical stability and storage services industry is growing as pharmaceutical companies outsource more to focus on their core strengths and use expert support for stability testing. Contract research organizations are increasing their services to include full stability testing, method development, validation, and regulatory support across the drug development lifecycle.
Digital tools and data analytics are enabling faster stability testing, utilizing predictive models and real-time monitoring to deliver greater value to clients. Increased demand for faster regulatory approvals is pushing companies to adopt outsourced stability services for quicker and more reliable data generation.
Pharmaceutical Stability And Storage Services Market: Challenges
Complex analytical requirements and technology integration issues
The pharmaceutical stability and storage services market is facing challenges in managing complex testing needs. Modern drugs have many active ingredients, additives, and packaging materials that need advanced testing methods to check stability and breakdown patterns correctly.
Creating and validating these methods for complex drugs takes a lot of time, is technically challenging, and needs expert skills and advanced tools. It is difficult to add new testing machines, software, and automation into laboratories that already have their own established systems. To follow changing rules, laboratories must constantly update their methods, check equipment, and keep proper records. It is also hard to train and keep skilled staff who can run detailed stability tests and understand the results properly.
Pharmaceutical Stability And Storage Services Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Pharmaceutical Stability And Storage Services Market |
| Market Size in 2024 | USD 3.53 Billion |
| Market Forecast in 2034 | USD 7.97 Billion |
| Growth Rate | CAGR of 8.50% |
| Number of Pages | 217 |
| Key Companies Covered | Eurofins Scientific, SGS SA, Intertek Group, Bureau Veritas, ALS Limited, Charles River Laboratories, Covance Inc., PPD Inc., IQVIA Holdings, Catalent Inc., West Pharmaceutical Services, Pace Analytical Services, Nelson Laboratories, Medistri SA, Boston Analytical, Microbac Laboratories, EAG Laboratories, Cambridge Consultants, Toxikon Corporation, BioPharma Services Inc., and others. |
| Segments Covered | By Service Type, By Application, By Distribution Channel, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Pharmaceutical Stability And Storage Services Market: Segmentation
The global pharmaceutical stability and storage services market is segmented into service type, application, distribution channel, end-user, and region.
Based on service type, the market is segregated into accelerated stability testing, real-time stability testing, forced degradation studies, and photostability testing. Accelerated stability testing leads the market due to shorter testing timelines, cost-effectiveness for preliminary stability assessment, and widespread use in early-stage drug development programs
Based on application, the pharmaceutical stability and storage services industry is classified into drug development, regulatory compliance, quality assurance, and shelf life determination. Regulatory compliance holds the largest market share due to mandatory stability testing requirements for drug approvals, standardized testing protocols, and critical importance for market authorization processes.
Based on the distribution channel, the pharmaceutical stability and storage services market is divided into contract research organizations, pharmaceutical companies, biotechnology firms, and third-party testing laboratories. Contract research organizations are expected to lead the market during the forecast period due to specialized expertise, comprehensive service offerings, and increasing outsourcing trends in pharmaceutical testing.
Based on the end-user, the market is segmented into large pharmaceutical companies, small and medium enterprises, generic drug manufacturers, and biosimilar developers. Large pharmaceutical companies lead the market share due to their extensive drug development pipelines, substantial testing requirements, and significant research and development investments in stability studies.
Pharmaceutical Stability And Storage Services Market: Regional Analysis
North America to lead the market
North America leads the pharmaceutical stability and storage services market due to its large pharmaceutical industry, advanced research infrastructure, and strict regulatory environment, which drives the need for comprehensive stability testing. The region accounts for approximately 45% of the global market, with the U.S. having many major pharmaceutical companies and CROs with specialized stability testing capabilities.
Research facilities in the region have state-of-the-art analytical equipment, controlled storage environments, and experienced scientific staff that attract clients from around the world. Well-established regulatory frameworks and harmonized guidelines mean predictable testing requirements and clear pathways to product approval. Strong IP protections and business-friendly environments support innovation and investment in pharmaceutical research and development. Academic partnerships and government funding initiatives promote advanced research in pharmaceutical stability and drug development technologies.
Europe is expected to show steady growth.
Europe is seeing steady growth in the pharmaceutical stability and storage services market as aligned regulations are making testing easier and improving market access across EU countries. Many European nations have robust pharmaceutical sectors and large generic drug production facilities that require stability testing support. Joint research by universities and industry is improving testing methods and analysis techniques.
Regulators in Europe are now employing risk-based testing approaches to optimize resource allocation while maintaining high product quality. EU countries are working together to share testing tools, laboratories, and expert knowledge. With more elderly people and rising healthcare demands, drug innovation is increasing and driving the need for stability testing.
Recent Market Developments:
- In January 2025, Pharmaceutical Testing Solutions International received approval for its advanced stability testing facility, featuring automated sample handling and real-time environmental monitoring capabilities.
Pharmaceutical Stability And Storage Services Market: Competitive Analysis
The global pharmaceutical stability and storage services market is led by players like:
- Eurofins Scientific
- SGS SA
- Intertek Group
- Bureau Veritas
- ALS Limited
- Charles River Laboratories
- Covance Inc.
- PPD Inc.
- IQVIA Holdings
- Catalent Inc.
- West Pharmaceutical Services
- Pace Analytical Services
- Nelson Laboratories
- Medistri SA
- Boston Analytical
- Microbac Laboratories
- EAG Laboratories
- Cambridge Consultants
- Toxikon Corporation
- BioPharma Services Inc.
The global pharmaceutical stability and storage services market is segmented as follows:
By Service Type
- Accelerated Stability Testing
- Real-Time Stability Testing
- Forced Degradation Studies
- Photostability Testing
By Application
- Drug Development
- Regulatory Compliance
- Quality Assurance
- Shelf Life Determination
By Distribution Channel
- Contract Research Organizations
- Pharmaceutical Companies
- Biotechnology Firms
- Third-Party Testing Laboratories
By End User
- Large Pharmaceutical Companies
- Small and Medium Enterprises
- Generic Drug Manufacturers
- Biosimilar Developers
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Pharmaceutical stability and storage services involve special testing to check how well products keep their quality, safety, and effectiveness over time in different environmental conditions.
The pharmaceutical stability and storage services market is expected to be driven by increasing pharmaceutical research and development activities, stringent regulatory requirements, growing outsourcing trends, and expanding specialty pharmaceutical development.
According to our study, the global pharmaceutical stability and storage services market was worth around USD 3.53 billion in 2024 and is predicted to grow to around USD 7.97 billion by 2034.
The CAGR value of the pharmaceutical stability and storage services market is expected to be around 8.50% during 2025-2034.
The global pharmaceutical stability and storage services market will register the highest revenue contribution from North America during the forecast period.
Key players in the pharmaceutical stability and storage services market include Eurofins Scientific, SGS SA, Intertek Group, Bureau Veritas, ALS Limited, Charles River Laboratories, Covance Inc., PPD Inc., IQVIA Holdings, Catalent Inc., West Pharmaceutical Services, Pace Analytical Services, Nelson Laboratories, Medistri SA, Boston Analytical, Microbac Laboratories, EAG Laboratories, Cambridge Consultants, Toxikon Corporation, and BioPharma Services Inc.
The report provides a comprehensive analysis of the pharmaceutical stability and storage services market, including an in-depth examination of market drivers, restraints, emerging trends, regional dynamics, and future growth prospects. It also examines the competitive dynamics, regulatory landscapes, service innovations, and technological factors that shape the pharmaceutical testing market ecosystem.
List of Contents
Pharmaceutical Stability And Storage ServicesIndustry Perspective:OverviewKey Insights:Growth DriversRestraintsOpportunitiesChallengesReport ScopeSegmentationRegional AnalysisRecent Market Developments:Competitive AnalysisThe global pharmaceutical stability and storage services market is segmented as follows:HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed