Preclinical CRO Treatment Market Demand, Size, Share & Forecast 2032

Preclinical CRO Treatment Market - by Type (Pharmacokinetics/Pharmacodynamics (PK/PD), Early Phase Development Services, Toxicology Testing, Laboratory Services, Clinic Research Services, Stability Testing, Physical Characterization, Raw Material Testing, Batch Release Testing, And Analytical Testing And Other Consulting Services), by Therapeutic Area (CNS Disorders, Oncology, Cardiovascular Diseases, Infectious Diseases, Respiratory Disorders, Immunological Disorders, Diabetes, Others), by End-User (Medical Device Companies, Pharmaceutical And Biopharmaceutical Companies, And Academic Institutes), and by Region: Global Industry Perspective, Comprehensive Analysis and Forecast 2024 - 2032
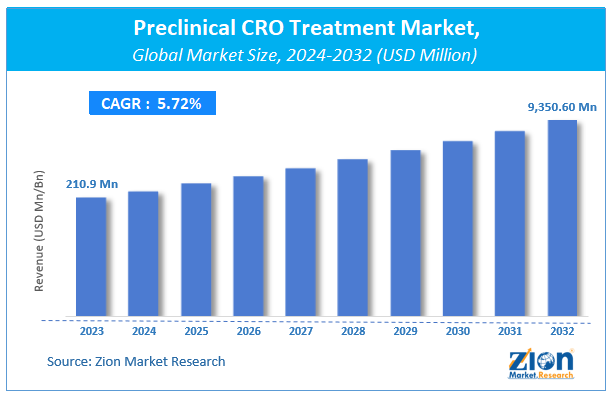
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 210.9 Million | USD 9,350.60 Million | 5.72% | 2023 |
Preclinical CRO Treatment Market: Industry Perspective
The global preclinical CRO treatment market size was worth around USD 210.9 million in 2023 and is predicted to grow to around USD 9,350.60 million by 2032 with a compound annual growth rate (CAGR) of roughly 5.72% between 2024 and 2032.
A preclinical CRO allows new developers of medical products (including electronics, medicines, biologics, and more) to show that they are safe and effective in live models. Rising spending on CRO services can propel the market growth, and also increase in the pressure to meet the R&D expenses is likely to boost the market in the forecast period.
Global Preclinical CRO Treatment Market: Overview
A preclinical CRO treatment is likely to offer the knowledge, experience, and skill needed to take a pharmaceutical medical device or product from the drawing board to distribution. The preclinical contract research organizations (CRO) sector has witnessed a period of tremendous development in the past, profiting from quickly growing (R&D) spending. Several pharmaceutical companies have lost revenue owing to growing patent expirations thus encouraging several manufacturers to outsource factors of the drug manufacturing method to CROs to decrease costs. Due to this, CROs are witnessing significant development.
Global Preclinical CRO Treatment Market: Growth Factors
The market is expected to witness profitable growth in the coming future due to an increase in R&D spending in the early stage of advancement along with the rise in a number of medicines in the preclinical stage. Hence, growth in outsourcing penetration is likely to contribute to the increasing demand and popularity of several life science companies, particularly for outsourced preclinical (CRO) treatment. Expansion in the number of rare/complex drugs that are entering into preclinical CRO treatment and increasing pressure to meet R&D expenses are projected to contribute to the increasing requirement for quality CROs, thus contributing to the preclinical CRO treatment market expansion.
Global Preclinical CRO Treatment Market: Segmentation
The global preclinical CRO treatment market has been divided into the following segments.
The global preclinical CRO treatment market is classified in terms of type, which is further divided into pharmacokinetics/pharmacodynamics (PK/PD), early phase development services, toxicology testing, laboratory services, clinic research services, stability testing, physical characterization, raw material testing, batch release testing, and analytical testing and other consulting services.
The global preclinical CRO treatment market is fragmented in terms of the therapeutic area which includes CNS disorders, oncology, cardiovascular diseases, infectious diseases, respiratory disorders, immunological disorders, and diabetes as well as other therapeutic areas.
The global preclinical CRO treatment market is segmented in terms of end-users into medical device companies, pharmaceutical and biopharmaceutical companies, and academic institutes.
Preclinical CRO Treatment Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Preclinical CRO Treatment Market |
| Market Size in 2023 | USD 210.9 Million |
| Market Forecast in 2032 | USD 9,350.60 Million |
| Growth Rate | CAGR of 5.72% |
| Number of Pages | 230 |
| Key Companies Covered | Paraxel International Corporation, Laboratory Corporation of America, Charles River Laboratories International, Inc., Eurofins Scientific, Envigo, Wuxi AppTec, PRA Health Science, Inc., Pharmaceutical Product Development, LLC., Medpace, Inc., and others. |
| Segments Covered | By Service, By Application, By End-User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Preclinical CRO Treatment Market: Regional Analysis
North America is expected to hold the largest market share in the global preclinical CRO market. It is anticipated to dominate and lead the global preclinical CRO treatment market in the coming future. The occurrence of established premature stage advancement CROs, for instance, LabCorp and Charles River Laboratories for improved quality of work, economic stability, established acumen & scientific experience, and logistic benefits to big life science companies are likely to be the few aspects that are expected to contribute to the domination of the region.
The varying industry models of rising costs of R&D and MNC outsourcing are projected to propel the global preclinical CRO treatment market in the Asia Pacific due to the cost-effectiveness of preclinical CRO treatment in countries such as China and India. Asia Pacific is anticipated to experience rapid development in the global preclinical CRO treatment market in the coming years.
Preclinical CRO Treatment Market: Competitive Analysis
The global preclinical CRO treatment market is dominated by players like:
- Paraxel International Corporation
- Laboratory Corporation of America
- Charles River Laboratories International, Inc.
- Eurofins Scientific
- Envigo
- Wuxi AppTec
- PRA Health Science, Inc.
- Pharmaceutical Product Development, LLC.
- Medpace, Inc.
The global preclinical CRO market is segmented as follows:
By Type Segment Analysis
- Pharmacokinetics/Pharmacodynamics (PK/PD)
- Early Phase Development Services
- Toxicology Testing
- Laboratory Services
- Clinic Research Services
- Stability Testing
- Physical Characterization
- Raw Material Testing
- Batch Release Testing
- Analytical Testing
- Others
By Therapeutic Area Segment Analysis
- CNS Disorders
- Oncology
- Cardiovascular Diseases
- Infectious Diseases
- Respiratory Disorders
- Immunological Disorders
- Diabetes
- Others
By End-User Segment Analysis
- Medical Device Companies
- Pharmaceutical And Biopharmaceutical Companies
- And Academic Institutes
By Regional Segment Analysis
-
North America
- The U.S.
- Europe
- The UK
- France
- Germany
- The Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- The Middle East and Africa
What Reports Provide
-
Full in-depth analysis of the parent market
- Important changes in market dynamics
- Segmentation details of the market
- Former, ongoing, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional markets
- Testimonials to companies in order to fortify their foothold in the market.
Table Of Content
Methodology
FrequentlyAsked Questions
A preclinical CRO treatment refers to a service provided by a Contract Research Organization (CRO) to perform tests on substances (potential drugs) in cells or animals before human trials can begin.
According to a study, the global preclinical CRO treatment market size was worth around USD 210.9 million in 2023 and is expected to reach USD 9,350.60 million by 2032.
The global preclinical CRO treatment market is expected to grow at a CAGR of 5.72% during the forecast period.
North America is expected to dominate the preclinical CRO treatment market over the forecast period.
Leading players in the global preclinical CRO treatment market include Paraxel International Corporation, Laboratory Corporation of America, Charles River Laboratories International, Inc., Eurofins Scientific, Envigo, Wuxi AppTec, PRA Health Science, Inc., Pharmaceutical Product Development, LLC., and Medpace, Inc., among others.
The preclinical CRO treatment market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTLE analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five forces analysis, and value chain analysis.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed