Contract Research Organization Market Size, Share, And Growth Report 2032

Contract Research Organization Market by Service Type (Clinical Research Services, Early-Phase Development Services, Laboratory Services, and Consulting Services), by Therapeutic Area (Oncology, Infectious Diseases, Central Nervous System (CNS) Disorders, Immunological Disorders, Cardiovascular Diseases, Respiratory Disorders, Diabetes, and Others), and by End-User (Pharmaceutical & Biopharmaceutical Companies, Medical Device Companies, and Others), By Region - Global And Regional Industry Overview, market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 76.36 Billion | USD 142.05 Billion | 7.14% | 2023 |
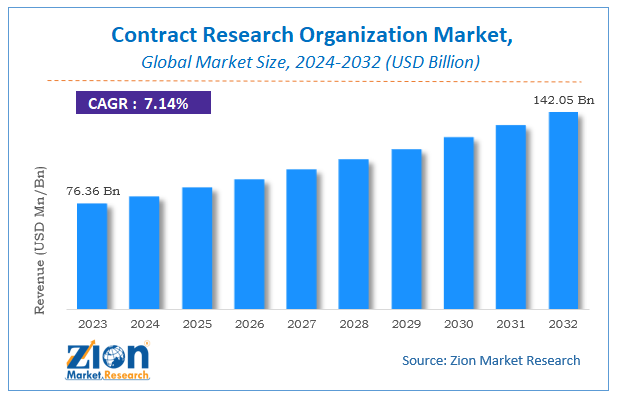
Contract Research Organization Market Size
The global Contract Research Organization Market size was worth around USD 76.36 billion in 2023 and is predicted to grow to around USD 142.05 billion by 2032 with a compound annual growth rate (CAGR) of roughly 7.14% between 2024 and 2032.
The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD billion). The report covers a forecast and an analysis of the Contract Research Organization Market on a global and regional level.
Contract Research Organization Market: Overview
Contract research organizations (CRO’s) have changed the way research is conducted in companies. CROs offer a wide variety of services these days, such as clinical trials, biopharmaceutical development, service type commercialization, assay development, drug discovery activities, etc. The increased demand for innovative devices and new therapies has led to a rise in the R&D budgets of these organizations. Most CRO’s are equipped with the required potential and skill to offer timely services for the completion of clinical trials.
Strict enforcement of legislation by regulators for better healthcare facilities and patent deaths are likely to fuel the global contract research organization market in the years ahead. As per the American pharmaceutical review, the CRO’s engagement in every clinical phase has increased over the recent years. Nearly 60% of companies in the global contract research organization market are appointing CROs and hiring the services of contract development and manufacturing organizations for Phase II study, which is a big jump from 2017. Emerging firms are outsourcing more than 50% of their services during Phase I study, while 70% of mid-sized companies outsource during Phase II. This has led to the development of the contract research organization market globally. Other factors that are anticipated to drive the contract research organization market include increasing number of clinical trials and growing R&D expenditure.
Contract Research Organization Market: Segmentation
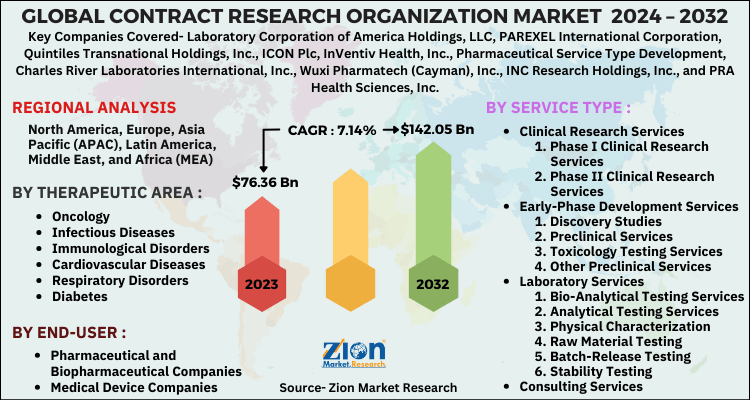
The study provides a decisive view of the contract research organization market by segmenting the market based on service type, therapeutic area, end-user, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
By service type, the market is segmented into contact clinical research services, laboratory services, early-phase development services, and consulting services. The clinical research services segment is further divided into phase I, phase II, phase III, and phase IV clinical research services. The early-phase development services segment is further divided into discovery studies, chemistry, manufacturing, and control, preclinical services, pharmacokinetics/pharmacodynamics, toxicology testing services, and other preclinical services. The laboratory services market is divided into stability testing, analytical testing services, batch-release testing, physical characterization, bio-analytical testing services, raw material testing, and other analytical testing services.
The therapeutic area segment includes infectious diseases, oncology, immunological disorders, cardiovascular diseases, central nervous system disorders, diabetes, respiratory disorders, and others.
By end-user, this market includes medical device companies, pharmaceutical and biopharmaceutical companies, and others.
Contract Research Organization Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Contract Research Organization Market |
| Market Size in 2023 | USD 76.36 Billion |
| Market Forecast in 2032 | USD 142.05 Billion |
| Growth Rate | CAGR of 7.14% |
| Number of Pages | 110 |
| Key Companies Covered | Laboratory Corporation of America Holdings, LLC, PAREXEL International Corporation, Quintiles Transnational Holdings, Inc., ICON Plc, InVentiv Health, Inc., Pharmaceutical Service Type Development, Charles River Laboratories International, Inc., Wuxi Pharmatech (Cayman), Inc., INC Research Holdings, Inc., and PRA Health Sciences, Inc |
| Segments Covered | By service type, By therapeutic area, By end-user and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Contract Research Organization Market: Regional Analysis
The regional segmentation includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and the Middle East and Africa with its further divided into major countries.
By region, North America dominated the contract research organization market in 2023, owing to the high standards of quality maintained by the pharmaceutical industry, the rapid growth of the biosimilars and biological market, increased government expenditure related to R&D expenses in healthcare, rising demand for outsourcing, and emerging biopharmaceutical industry. Europe is likely to be the second leading contract research organization market globally in the years ahead, owing to the increasing investments made by the key players in R&D activities and continuous innovation in medical technology. The Asia Pacific region has showcased significant growth potential in this market, due to the increasing prevalence of diseases and the high availability of subjects for preclinical and clinical trials.
Contract Research Organization Market: Competitive Players
Some leading players of the global contract research organization market include:
- Laboratory Corporation of America Holdings LLC
- PAREXEL International Corporation
- Quintiles Transnational Holdings Inc.
- ICON Plc
- InVentiv Health Inc.
- Pharmaceutical Service Type Development
- Charles River Laboratories International Inc.
- Wuxi Pharmatech (Cayman) Inc.
- INC Research Holdings Inc.
- PRA Health Sciences Inc.
The Global Contract Research Organization Market is segmented as follows:
Global Contract Research Organization Market: Service Type Analysis
- Clinical Research Services
- Phase I Clinical Research Services
- Phase II Clinical Research Services
- Phase III Clinical Research Services
- Phase IV Clinical Research Services
- Early-Phase Development Services
- Discovery Studies
- Chemistry, Manufacturing, and Control (CMC)
- Preclinical Services
- Pharmacokinetics/Pharmacodynamics (PK/PD)
- Toxicology Testing Services
- Other Preclinical Services
- Laboratory Services
- Bio-Analytical Testing Services
- Analytical Testing Services
- Physical Characterization
- Raw Material Testing
- Batch-Release Testing
- Stability Testing
- Other Analytical Testing Services
- Consulting Services
Global Contract Research Organization Market: Therapeutic Area Analysis
- Oncology
- Infectious Diseases
- Central Nervous System (CNS) Disorders
- Immunological Disorders
- Cardiovascular Diseases
- Respiratory Disorders
- Diabetes
- Others
Global Contract Research Organization Market: End-User Analysis
- Pharmaceutical and Biopharmaceutical Companies
- Medical Device Companies
- Others
Global Contract Research Organization Market: Regional Analysis
- North America
- U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Contract research organizations (CRO’s) have changed the way research is conducted in companies. CROs offer a wide variety of services these days, such as clinical trials, biopharmaceutical development, service type commercialization, assay development, drug discovery activities, etc.
According to study, the Contract Research Organization Market size was worth around USD 76.36 billion in 2023 and is predicted to grow to around USD 142.05 billion by 2032.
The CAGR value of Contract Research Organization Market is expected to be around 7.14% during 2024-2032.
North America has been leading the Contract Research Organization Market and is anticipated to continue on the dominant position in the years to come.
The Contract Research Organization Market is led by players like Laboratory Corporation of America Holdings LLC, PAREXEL International Corporation, Quintiles Transnational Holdings Inc., ICON Plc, InVentiv Health Inc., Pharmaceutical Service Type Development, Charles River Laboratories International Inc., Wuxi Pharmatech (Cayman) Inc., INC Research Holdings Inc., and PRA Health Sciences Inc.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed