Cell Based Cartilage Repair Regeneration Market Size, Share, Trends, Growth 2034

Cell Based Cartilage Repair Regeneration Market By Therapy Type (Autologous Cell Therapy, Allogeneic Cell Therapy, and Others), By Mode of Administration (Intra-Articular Injection, Scaffold Implantation, Surgical Implantation), By End-User (Hospitals and Clinics, Research Institutions and Biotechnology, Pharmaceutical Companies), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 748.89 Million | USD 1132.0 Million | 5.30% | 2024 |
Cell Based Cartilage Repair Regeneration Industry Perspective:
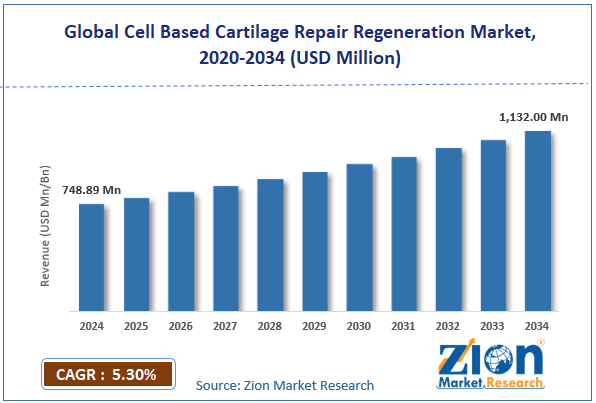
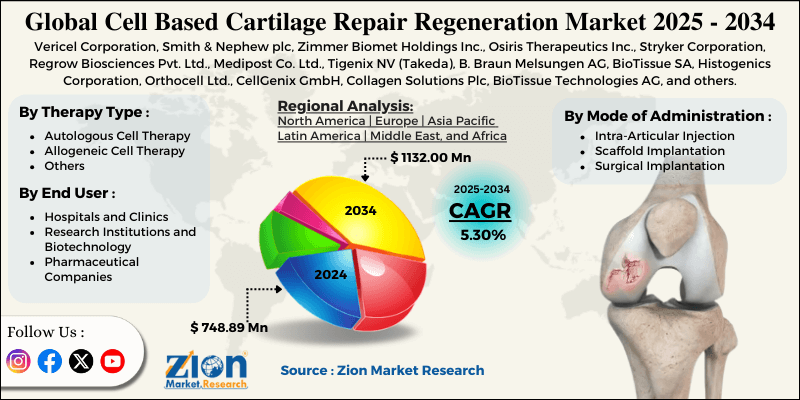
The global cell based cartilage repair regeneration market size was worth around USD 748.89 million in 2024 and is predicted to grow to around USD 1132.00 million by 2034, with a compound annual growth rate (CAGR) of roughly 5.30% between 2025 and 2034.
Key Insights:
- As per the analysis shared by our research analyst, the global cell based cartilage repair regeneration market is estimated to grow annually at a CAGR of around 5.30% over the forecast period (2025-2034)
- In terms of revenue, the global cell based cartilage repair regeneration market size was valued at around USD 748.89 million in 2024 and is projected to reach USD 1132.00 million by 2034.
- The cell based cartilage repair regeneration market is projected to grow significantly owing to the growing demand for minimally invasive surgical procedures, supportive government funding and initiatives for cell therapy research, and surging cases of cartilage injuries and osteoarthritis.
- Based on therapy type, the autologous cell therapy segment is expected to lead the market, while the allogeneic cell therapy segment is expected to grow considerably.
- Based on mode of administration, the surgical implantation segment is the dominating segment, while the intra-articular injection segment is projected to witness sizeable revenue over the forecast period.
- Based on end user, the hospitals and clinics segment is expected to lead the market compared to the research institutions and biotechnology segment.
- Based on region, North America is projected to dominate the global market during the estimated period, followed by Europe.
Cell Based Cartilage Repair Regeneration Market: Overview
Cell-based cartilage repair and regeneration is an improved therapeutic approach that uses living cells, such as stem cells or chondrocytes, to restore damaged cartilage in joints. This approach regenerates hyaline-like cartilage, enhancing joint function and reducing pain, especially in conditions like sports injuries or osteoarthritis. The global cell based cartilage repair regeneration market is projected to witness substantial growth driven by the growing sports injuries and active lifestyle trends, improvements in stem cell technology and regenerative medicine, and growing demand for minimally invasive surgeries. Sports injuries result in millions of cartilage damage cases per year, mainly among fitness enthusiasts and young athletes. These injuries create a significant demand for regenerative therapies that restore mobility and ensure quicker recovery. Advanced cell-based solutions are progressing as a highly favored treatment option in these cases.
Also, continuous advancements in stem cell research, scaffold materials, and tissue engineering have transformed cartilage regeneration methods. Modern bioactive scaffolds blended with stem cells offer superior durability and integration. This accelerates industry adoption and improves patient outcomes. Also, patients vastly prefer minimally invasive procedures because of short recovery times and fewer complications. Cell-based therapies, mainly injectable cell treatments, support this trend. This preference is aiding healthcare providers in adopting advanced regenerative solutions.
Although drivers exist, the global market is challenged by factors like the high cost of treatment and the lack of skilled professionals. Cell-based cartilage repair procedures like ACI are expensive. This cost usually ranges between USD 10,000 and USD 40,000, making it unaffordable for several patients. This pricing challenge limits adoption, mainly in the developing markets. Additionally, these advanced procedures require surgical proficiency and access to well-equipped facilities.
The lack of trained professionals in several regions hinders the adoption. Even so, the global cell-based cartilage repair regeneration industry is well-positioned due to the integration with scaffold technology, 3D bioprinting, and personalized medicine and gene editing applications. Combining cell-based regeneration with 3D bioprinting may deliver customized cartilage implants. This solution enables precise defect repair and enhanced clinical outcomes.
Future advancements in this domain can majorly impact the market progress. Also, innovations in genetic engineering and CRISPR allow the development of personalized cell-based therapies. They can enhance tissue regeneration and decrease graft failure risks. Precision medicine is a key opportunity for next-gen treatments.
Cell Based Cartilage Repair Regeneration Market: Market Dynamics
Growth Drivers
How is the shift in clinical practice spurring the cell based cartilage repair regeneration market growth?
Patients and orthopedic surgeons are actively choosing procedures that preserve natural joint structures instead of resorting to total joint replacements. Minimally invasive methods, comprising arthroscopic implantation and injectable therapies, are progressing and being favored mainly because of a lower complication rate and fast recovery time. These procedures are primarily appealing to the athletic and younger patients, who avoid prosthetics and seek mobility. The move in practice patterns has created a robust pull for advanced cartilage repair solutions.
Clinical pipeline expansion, growing R&D, and capital flow fuel the market growth
The worldwide cell based cartilage repair regeneration market is experiencing a rise in research investments and clinical trials, denoting strong innovation momentum. By mid-2024, multiple Phase I/II and many Phase III studies were ongoing across the globe, testing allogenic and autologous solutions. Strategic associations between biotech companies and academic institutions have boosted translational research. Increased venture capital funding and corporate investments are offering a strong infrastructure for new entrants and well-established players. This dynamic pipeline increases the likelihood of novel product launches and accelerates regulatory approvals.
Restraints
How does technical and surgical complexity negatively impact the cell based cartilage repair regeneration market development?
Cell-based cartilage repair procedures are technically demanding, needing highly proficient orthopedic surgeons trained in managing injectable matrices and cell implants. The lack of standardized training programs and learning curve restricts the number of specialists capable of performing these procedures worldwide. This lack of expertise decreases the number of certified centers offering modernized regenerative solutions, especially in Latin America and the Asia Pacific.
Opportunities
How does the integration of advanced biomaterials and 3D bioprinting create promising avenues for cell based cartilage repair regeneration industry growth?
3D bioprinting technology assimilated with bioactive scaffolds is transforming personalized cartilage repair solutions. This modernization allows precise patient-specific implants that imitate natural cartilage architecture, enhancing outcomes. The interaction between biomaterials, bioprinting, and stem cells offers a base for next-generation products. With the progress of these technologies, companies that integrate them into their portfolios are poised to gain a significant competitive benefit, impacting the progress of the global cell based cartilage repair regeneration industry.
Challenges
Lack of comparative data and long-term durability restricts the market growth
While short to mid-term clinical outcomes for cell-based cartilage repair are promising, the lack of 10+ year durability data still restricts payer confidence. Surgeons are also looking for strong head-to-head comparisons with conventional to conventional techniques like osteochondral and microfracture grafts. Without long-term real-world evidence, insurers are reluctant to offer exhaustive reimbursement, and hospitals experience adoption risks. This evidence gap is still a key challenge for achieving mainstream adoption.
Cell Based Cartilage Repair Regeneration Market: Segmentation
The global cell based cartilage repair regeneration market is segmented based on therapy type, mode of administration, end user, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2023 to 2030.
Based on therapy type, the global cell based cartilage repair regeneration industry is divided into autologous cell therapy, allogeneic cell therapy, and others. The autologous cell therapy segment registered a substantial share of the market owing to its high clinical success and lower risk of immune rejection. Procedures like Autologous Chondrocyte Implantation are broadly considered a standard therapy for cartilage repair. The therapy uses the cells of the patient, assuring better compatibility and long-term outcomes. Its effectiveness in treating focal cartilage defects and sports injuries increases its preference among orthopedic professionals.
Based on mode of administration, the global cell based cartilage repair regeneration market is segmented into intra-articular injection, scaffold implantation, and surgical implantation. The surgical implantation holds a dominating share of the market. The method enables the precise placement of cells or tissue-engineered constructs straight into the cartilage defect. It is broadly used in procedures like ACI, which remains the gold standard for repairing complex or extensive cartilage injuries. Despite its invasiveness, its long-term clinical outcomes and high success rate increase its adoption and preference.
Based on end user, the global market is segmented as hospitals and clinics, research institutions and biotechnology, and pharmaceutical companies. The hospitals and clinics segment holds leadership in the market since these facilities manage the majority of surgical procedures, comprising ACI and stem cell-based therapies. Their availability of skilled orthopedic surgeons, advanced infrastructure, and post-operative care capabilities increases their popularity in these primary centers for treatment. Rising patient choice for specialized orthopedic hospitals reinforces the segmental dominance.
The Regional, this segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America,and the Middle East and Africa.
Cell Based Cartilage Repair Regeneration Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Cell Based Cartilage Repair Regeneration Market |
| Market Size in 2024 | USD 748.89 Million |
| Market Forecast in 2034 | USD 1132.00 Million |
| Growth Rate | CAGR of 5.30% |
| Number of Pages | 218 |
| Key Companies Covered | Vericel Corporation, Smith & Nephew plc, Zimmer Biomet Holdings Inc., Osiris Therapeutics Inc., Stryker Corporation, Regrow Biosciences Pvt. Ltd., Medipost Co. Ltd., Tigenix NV (Takeda), B. Braun Melsungen AG, BioTissue SA, Histogenics Corporation, Orthocell Ltd., CellGenix GmbH, Collagen Solutions Plc, BioTissue Technologies AG, and others. |
| Segments Covered | By Therapy Type, By Mode of Administration, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Cell Based Cartilage Repair Regeneration Market: Regional Analysis
Why is North America outperforming other regions in the global Cell Based Cartilage Repair Regeneration Market?
North America is likely to sustain its leadership in the cell based cartilage repair regeneration market due to the strong healthcare infrastructure and availability of professionals, technological improvements, high R&D, a supportive regulatory framework, and faster product approvals. The region benefits from well-developed healthcare systems with advanced surgical facilities and trained and experienced orthopedic surgeons. This ecosystem aligns with the broader adoption of complex regenerative techniques like stem cell-based therapies and ACI.
Also, North America is a forerunner in regenerative medicine advancement, facilitated by significant funding from both public and private sectors. Continuous improvements in stem cell applications and tissue engineering further boost the region's prominence. The FDA offers structured rules for cell-based therapy approvals, allowing companies to commercialize products efficiently. Programs like RMAT (Regenerative Medicine Advanced Therapy) designation fast-track approval for advanced treatments. This regulatory backing motivates the introduction of advanced cartilage repair products in the North American industry.
Europe continues to secure the second-highest share in the cell based cartilage repair regeneration industry owing to the growing cases of joint disorders and osteoarthritis, advanced healthcare infrastructure, and supportive regulatory architecture for advanced therapies. Europe holds a central pressure of osteoarthritis, with over 40 million individuals suffering in the European Union. The sedentary lifestyles and aging population add to an increasing number of cartilage-associated issues. The rising patient pool fuels stronger demand for regenerative therapies. Europe offers a well-established healthcare system with sports medicine and specialized orthopedic centers.
Economies like France and Germany have some of the leading rates of cartilage repair procedures in Europe. This ecosystem allows smooth adoption of advanced cell-based therapies. Additionally, the EMA has created specific pathways for Advanced Therapy Medicinal Products, which comprise cell-based treatments. These regulations simplify approvals and boost innovation in regenerative medicine, aiding the regional dominance.
Cell Based Cartilage Repair Regeneration Market: Competitive Analysis
The leading players in the global cell based cartilage repair regeneration market are:
- Vericel Corporation
- Smith & Nephew plc
- Zimmer Biomet Holdings Inc.
- Osiris Therapeutics Inc.
- Stryker Corporation
- Regrow Biosciences Pvt. Ltd.
- Medipost Co. Ltd.
- Tigenix NV (Takeda)
- B. Braun Melsungen AG
- BioTissue SA
- Histogenics Corporation
- Orthocell Ltd.
- CellGenix GmbH
- Collagen Solutions Plc
- BioTissue Technologies AG
Cell Based Cartilage Repair Regeneration Market: Key Market Trends
Integration of Tissue Engineering and 3D Bioprinting:
3D bioprinting technology is being combined with cell therapies to create tailored cartilage implants. These implants enable better integration and faster healing than traditional scaffolds. This advancement is anticipated to transform the industry by offering highly personalized regenerative solutions.
Increased regulatory support for advanced therapies:
Regulatory agencies like the EMA and FDA have launched fast-track approval pathways for regenerative medicine products. Programs like ATMP classification in Europe and RMAT in the United States speed up commercialization and encourage innovation. This supportive environment is boosting the availability of advanced cell-based cartilage repair solutions.
The global cell based cartilage repair regeneration market is segmented as follows:
By Therapy Type
- Autologous Cell Therapy
- Allogeneic Cell Therapy
- Others
By Mode of Administration
- Intra-Articular Injection
- Scaffold Implantation
- Surgical Implantation
By End User
- Hospitals and Clinics
- Research Institutions and Biotechnology
- Pharmaceutical Companies
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Cell-based cartilage repair and regeneration is an improved therapeutic approach that uses living cells, such as stem cells or chondrocytes, to restore damaged cartilage in joints. This approach regenerates hyaline-like cartilage, enhancing joint function and reducing pain, especially in conditions like sports injuries or osteoarthritis.
The global cell based cartilage repair regeneration market is projected to grow due to the growing geriatric population worldwide, rising trauma cases and sports-related injuries, and improvements in biomaterials and tissue engineering.
According to study, the global cell based cartilage repair regeneration market size was worth around USD 748.89 million in 2024 and is predicted to grow to around USD 1132.00 million by 2034.
What will be the CAGR value of the cell based cartilage repair regeneration market during 2025-2034?
The CAGR value of the cell based cartilage repair regeneration market is expected to be around 5.30% during 2025-2034.
Technological advancements like advanced biomaterial scaffolds, 3D bioprinting, and stem cell engineering are enhancing treatment precision, improving clinical outcomes, and augmenting the adoption of cell-based cartilage repair solutions.
The market is witnessing high treatment costs with gradual price reductions fueled by increased competition, technological innovations, and surging adoption of scalable allogeneic therapies.
North America is expected to lead the global cell based cartilage repair regeneration market during the forecast period.
The key players profiled in the global cell based cartilage repair regeneration market include Vericel Corporation, Smith & Nephew plc, Zimmer Biomet Holdings, Inc., Osiris Therapeutics, Inc., Stryker Corporation, Regrow Biosciences Pvt. Ltd., Medipost Co., Ltd., Tigenix NV (Takeda), B. Braun Melsungen AG, BioTissue SA, Histogenics Corporation, Orthocell Ltd., CellGenix GmbH, Collagen Solutions Plc, and BioTissue Technologies AG.
Stakeholders should focus on strategic partnerships, R&D innovation, regulatory compliance, cost optimization, and expansion into emerging markets to maintain competitiveness in the cell based cartilage repair regeneration market.
The report examines key aspects of the cell based cartilage repair regeneration market, including a detailed analysis of existing growth factors and restraints, as well as an examination of future growth opportunities and challenges that will impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed