Viral Diagnostic Test Kits Market Size, Share, Trends, Growth 2032

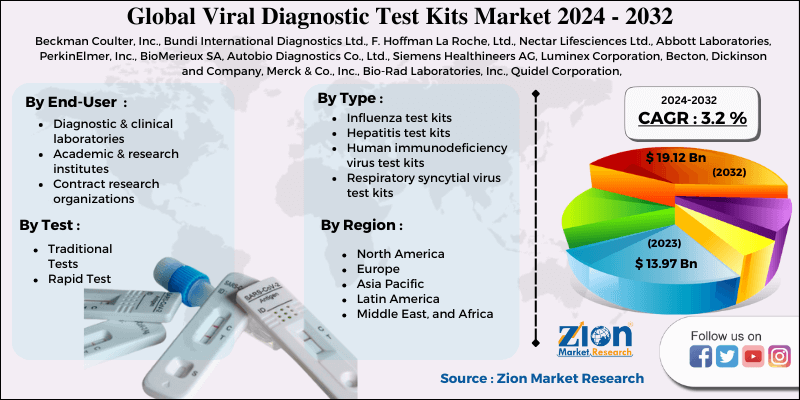
Viral Diagnostic Test Kits Market Size By Type (Influenza test kits, Hepatitis test kits, Human immunodeficiency virus test kits, and Respiratory syncytial virus test kits), By Test (Traditional Tests and Rapid Tests), By End-User (Diagnostic & clinical laboratories, Academic & research institutes, and Contract research organizations), And By Region- Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032-
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 13.97 Billion | USD 19.12 Billion | 3.2% | 2023 |
Viral Diagnostic Test Kits Market Insights
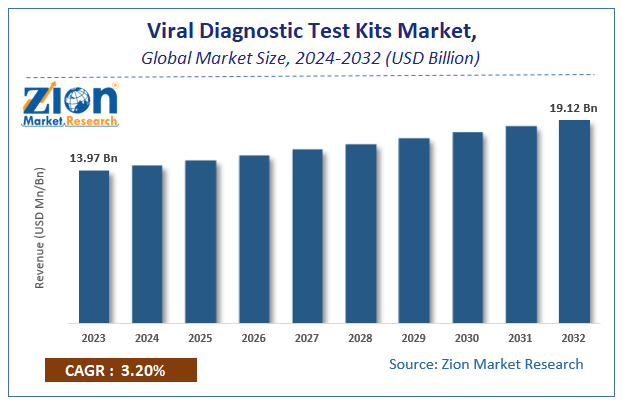
According to a report from Zion Market Research, the global Viral Diagnostic Test Kits Market was valued at USD 13.97 Billion in 2023 and is projected to hit USD 19.12 Billion by 2032, with a compound annual growth rate (CAGR) of 3.2% during the forecast period 2024-2032. This report explores market strengths, weakness, opportunities, and threats. It also provides valuable insights into the market's growth drivers, challenges, and the future prospects that may emerge in the Viral Diagnostic Test Kits Market industry over the next decade.
Viral Diagnostic Test Kits Market: Overview
One of the most proficient methods for preventing the spread of SARS-CoV-2 is to create accurate rapid diagnostic tests. Apparently, there are many serological, imaging, and molecular techniques as well as various diagnostic kits for diagnosing infection in clinics. In addition to this, the SARS-CoV-2 determining methods used by labs and firms include molecular methods having the ability to detect viral RNA sequencing, rapid diagnostic tests for identifying virus presence depending on antigens, and imaging methods for determining changes occurring in lungs.
In the molecular approach, PCR and high throughput sequencing are used for identifying virus presence in respiratory samples. Radio imaging methods such as CT-scan are utilized for measuring the level of infection. However, rapid diagnostic tests are used prominently as they are time-savvy and accurate. They make use of antigens or antibodies for determining virus presence in individuals.
Apart from this, antibody-based testing methods including ELISA are utilized for determining antibodies in samples of patients and assessing whether the subject was infected earlier or not. Furthermore, antibody tests are also done by using blood samples as well as samples of serum and plasma. Furthermore, antigen tests help in detecting the viral antigen presence in respiratory samples along with identifying active infection. Furthermore, viral diagnostic test kits are used for performing these antibody or antigen tests for detecting the virus in the human body. This will steer the viral diagnostic test kits market size.
Viral Diagnostic Test Kits Market: Growth Dynamics
With the onset of the COVID pandemic, the market for viral diagnostic test kits is anticipated to gain traction over the upcoming years. Apart from this, swine flu and HIV has further added to the demand for viral diagnostic test kits in recent years, thereby driving business trends. A prominent surge in viral infections including respiratory syncytial virus and hepatitis is projected to steer viral diagnostic test kits market size in the years ahead. In addition to this, an outbreak of new viral strains along with the inception of virus infections including Ebola and hemorrhagic fever will create humungous product demand within the next couple of years. Nonetheless, the huge costs of viral diagnostic test kits can restrict the growth of the viral diagnostic test kits industry over the upcoming years.
Moreover, the launching of new rapid test kits and laws favoring the use of viral diagnostic test kits along with the rapid spread of virus-like COVID-19 among the population will chart a profitable growth map for the viral diagnostic test kits industry within the next couple of years. Apart from this, firms are trying to develop new kits to determine the presence of viruses such as COVID-19.
Citing an instance, on 9th April 2021, LUCIRA – a medical tech firm producing new infectious ailment test kits, announced the successful detection of a double mutant COVID-19 variant through its viral diagnostic test kit referred to as LUCIRA molecular diagnostic test kit. In addition to this, earlier in March 2021, U.S.-based firm Cepheid is likely to produce point-of-care COVID-19 diagnostic test kits in the Indian sub-continent. Such strategic moves can lay strong foundations for viral diagnostic test kits market growth across the globe in the coming years.
Viral Diagnostic Test Kits Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Viral Diagnostic Test Kits Market |
| Market Size in 2023 | USD 13.97 Billion |
| Market Forecast in 2032 | USD 19.12 Billion |
| Growth Rate | CAGR of 3.2% |
| Number of Pages | 114 |
| Key Companies Covered | Beckman Coulter, Inc., Bundi International Diagnostics Ltd., F. Hoffman La Roche, Ltd., Nectar Lifesciences Ltd., Abbott Laboratories, PerkinElmer, Inc., BioMerieux SA, Autobio Diagnostics Co., Ltd., Siemens Healthineers AG, Luminex Corporation, Becton, Dickinson and Company, Merck & Co., Inc., Bio-Rad Laboratories, Inc., Quidel Corporation, CerTest Biotec, S.L., Qiagen NV, CorisBioconcept SPRL, Hologic Inc., Mylan NV, and Thermo Fisher Scientific, Inc. |
| Segments Covered | By Test, By Type, By End Use, And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Viral Diagnostic Test Kits Market: Regional Landscape
North America To Continue Market Domination In Near Future
The growth of the market in the North American sub-continent over the assessment period is attributed to increasing in cases of COVID in countries such as the U.S. Apart from this, the production of new test kits along with favorable government policies over the manufacture of the products will create strong growth platform for viral diagnostic test kits industry in the sub-continent over the expected timespan. The presence of giant firms in the region will prop up the business landscape in the next decade, thereby further adding to regional market value.
Viral Diagnostic Test Kits Market: Competitive Landscape
Key participants profiled in the report are
- Beckman Coulter. Inc.
- Bundi International Diagnostics Ltd.
- F. Hoffman La Roche. Ltd.
- Nectar Lifesciences Ltd.
- Abbott Laboratories
- PerkinElmer. Inc.
- BioMerieux SA
- Autobio Diagnostics Co.Ltd.
- Siemens Healthineers AG
- Luminex Corporation
- Becton
- Dickinson and Company
- Merck & Co.. Inc.
- Bio-Rad Laboratories. Inc.
- Quidel Corporation
- CerTest Biotec
- S.L.
- Qiagen NV
- CorisBioconcept SPRL
- Hologic Inc.
- Mylan NV
- Thermo Fisher Scientific. Inc.
The global Viral Diagnostic Test Kits Market is segmented as follows:
By Test
- Traditional Tests
- Rapid Test
By Type
- Influenza test kits
- Hepatitis test kits
- Human immunodeficiency virus test kits
- Respiratory syncytial virus test kits
By End-User
- Diagnostic & clinical laboratories
- Academic & research institutes
- Contract research organizations
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Medical tools or devices used to identify virus presence in a patient's body are viral diagnostic test kits. Diagnosing viral infections depends on these kits, which also enable doctors to pinpoint the particular virus causing a disease and direct suitable therapy or intervention. From hospitals to home-based testing, they find application in many healthcare environments.
Rapid antigen tests, home testing kits, and molecular diagnostics—among other constant advancements in test sensitivity, speed, and ease of use—are making testing more reliable and accessible.
According to a report from Zion Market Research, the global Viral Diagnostic Test Kits Market was valued at USD 13.97 Billion in 2023 and is projected to hit USD 19.12 Billion by 2032.
According to a report from Zion Market Research, the global Viral Diagnostic Test Kits Market a compound annual growth rate (CAGR) of 3.2% during the forecast period 2024-2032.
The growth of the market in the North American sub-continent over the assessment period is attributed to increasing in cases of COVID in countries such as the U.S. Apart from this, the production of new test kits along with favorable government policies over the manufacture of the products will create strong growth platform for viral diagnostic test kits industry in the sub-continent over the expected timespan.
Beckman Coulter, Inc., Bundi International Diagnostics Ltd., F. Hoffman La Roche, Ltd., Nectar Lifesciences Ltd., Abbott Laboratories, PerkinElmer, Inc., BioMerieux SA, Autobio Diagnostics Co., Ltd., Siemens Healthineers AG, Luminex Corporation, Becton, Dickinson and Company, Merck & Co., Inc., Bio-Rad Laboratories, Inc., Quidel Corporation, CerTest Biotec, S.L., Qiagen NV, CorisBioconcept SPRL, Hologic Inc., Mylan NV, and Thermo Fisher Scientific, Inc.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed