Nucleic Acid Amplification Testing (NAAT) Market Size, Share, Trends, Growth 2034

Nucleic Acid Amplification Testing (NAAT) Market By Product Type (Polymerase Chain Reaction (PCR), Loop-mediated Isothermal Amplification (LAMP), Transcription-mediated Amplification (TMA), Strand Displacement Amplification (SDA), and Nucleic Acid Sequence-based Amplification (NASBA)), By Technology (Real-time PCR, Digital PCR, Reverse Transcription PCR, Multiplex PCR, Nested PCR, and Others), By Application (Infectious Disease Testing, Genetic Testing, Oncology Testing, Blood Screening, Prenatal Testing, Forensic Testing), By End-User (Hospitals and Clinics, Diagnostic Laboratories, Blood Banks, Research Institutions, Academic Centers, Home Care Settings), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 9.14 Billion | USD 28.90 Billion | 12.20% | 2024 |
Nucleic Acid Amplification Testing (NAAT) Industry Perspective:
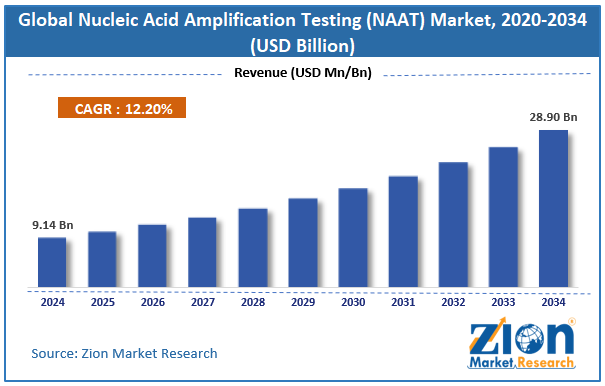
The global nucleic acid amplification testing (NAAT) market size was worth approximately USD 9.14 billion in 2024 and is projected to grow to around USD 28.90 billion by 2034, with a compound annual growth rate (CAGR) of roughly 12.20% between 2025 and 2034.
Key Insights:
- As per the analysis shared by our research analyst, the global nucleic acid amplification testing market is estimated to grow annually at a CAGR of around 12.20% over the forecast period (2025-2034).
- In terms of revenue, the global nucleic acid amplification testing market size was valued at approximately USD 9.14 billion in 2024 and is projected to reach USD 28.90 billion by 2034.
- The nucleic acid amplification testing market is projected to grow significantly due to the increasing prevalence of infectious diseases and rising demand for rapid diagnostic solutions.
- Based on product type, the polymerase chain reaction (PCR) segment is expected to lead the nucleic acid amplification testing market. In contrast, the loop-mediated isothermal amplification (LAMP) segment is anticipated to experience significant growth.
- Based on technology, the real-time PCR segment is expected to lead the nucleic acid amplification testing market, while the digital PCR segment is anticipated to witness notable growth.
- Based on application, the infectious disease testing segment is the dominating segment, while the oncology testing segment is projected to witness sizeable revenue over the forecast period.
- Based on end-user, the hospitals and clinics segment is expected to lead the market compared to the home care settings segment.
- Based on region, North America is projected to dominate the global nucleic acid amplification testing market during the estimated period, followed by Europe.
Nucleic Acid Amplification Testing (NAAT) Market: Overview
Nucleic Acid Amplification Testing (NAAT) is a molecular diagnostic method used to amplify and detect specific DNA or RNA sequences to identify pathogens, genetic changes, and cellular abnormalities with high precision and sensitivity. These advanced testing methods allow healthcare professionals to diagnose infectious diseases, track genetic conditions, and detect cancer markers at the molecular level, providing quick results with high accuracy.
Modern NAAT platforms include multiple amplification technologies such as polymerase chain reaction (PCR), isothermal amplification methods, and transcription-mediated systems, all of which can process multiple samples at once. These platforms are able to detect very small amounts of genetic material from bacteria, viruses, and human cells, which makes them valuable for early disease detection and public health surveillance. Manufacturers are focusing on creating easy-to-use interfaces, reducing testing time, and improving multiplexing capabilities while still ensuring strict quality standards and regulatory compliance.
The growing emphasis on precision medicine and personalized healthcare is expected to drive growth in the nucleic acid amplification testing market throughout the forecast period.
Nucleic Acid Amplification Testing (NAAT) Market Dynamics
Growth Drivers
Increasing prevalence of infectious diseases and emerging pathogen threats
The nucleic acid amplification testing market is growing fast as healthcare systems worldwide face challenges from disease outbreaks, drug resistance, and new pathogen threats that need accurate molecular detection. Doctors and hospitals are turning to NAAT technologies for quick and accurate diagnosis of bacterial, viral, and fungal infections that older culture methods cannot detect well. The rising number of hospital-related infections is creating demand for fast diagnostic tools that support better treatment choices and enhanced infection control.
New diseases and pandemic readiness programs are driving demand for flexible NAAT systems that allow quick test development and use. The rise of antibiotic-resistant bacteria makes advanced testing crucial for identifying resistance genes and informing the most effective treatments. Global travel and urban growth are spreading diseases faster, making rapid molecular testing essential for public health tracking and outbreak control.
How are advancements in automation and point-of-care testing capabilities propelling the nucleic acid amplification testing market growth?
The global nucleic acid amplification testing industry is also growing as companies design more automated systems and portable devices that make molecular testing easier and closer to patients. New systems offer complete sample-to-result testing that removes manual steps, lowers contamination risks, and gives consistent results even with different operators. Point-of-care NAAT devices bring rapid testing to emergency rooms, clinics, and remote locations where laboratory services are limited. Robotics and artificial intelligence are boosting testing speed, accuracy, and result interpretation while reducing human error.
Smaller versions of amplification technologies are making testing possible in low-resource settings and even at home for some uses. Cloud-based systems help in the sharing of real-time results and the tracking of disease patterns. These improvements make NAAT more widely available by reducing infrastructure needs, streamlining staff training, and simplifying operations, while maintaining high performance standards.
Restraints
High equipment costs and technical complexity requirements
A major challenge for the nucleic acid amplification testing market is the high cost of equipment, laboratory setup, and ongoing service needs. This makes advanced testing expensive for many small hospitals and laboratories with limited budgets. Running complex NAAT systems requires skilled staff who can operate machines, read results, and solve issues, which creates training and education needs. Quality checks and regulatory rules add more cost and operational challenges, especially for facilities with fewer resources. Preparing samples and preventing contamination require special laboratory designs and strict workflow steps, adding to costs. The fast pace of technology change also makes administrators worry that expensive machines may become outdated in a few years, making them hesitant to invest.
Opportunities
How are expanding applications in personalized medicine and companion diagnostics creating opportunities for the market?
The nucleic acid amplification testing market is growing beyond infectious disease testing into areas like personalized medicine, pharmacogenomics, and companion diagnostics that help doctors choose the best treatments. Cancer testing uses NAAT to find genetic mutations, monitor minimal residual disease, and check treatment response with liquid biopsies. Pharmacogenomic testing uses molecular methods to study how patients metabolize drugs, helping adjust doses for optimal safety and effectiveness. Prenatal and reproductive health testing is expanding with NAAT, offering safer and more accurate non-invasive screening.
Genetic counseling and hereditary disease screening are driving the development of new applications for molecular diagnostics. Outside healthcare, NAAT is also being applied in agriculture and veterinary medicine. Direct-to-consumer testing and wellness monitoring are opening opportunities for simple, user-friendly NAAT platforms. These applications are driving new solutions with faster results, more test combinations, and lower costs.
Challenges
How are regulatory complexity and reimbursement limitations limiting the growth of the market?
The nucleic acid amplification testing market faces challenges from complex regulations and varying reimbursement rules across different countries. Approving new diagnostic tests requires long and costly validation and submission processes. Different approval systems for lab-developed tests, FDA-cleared devices, and companion diagnostics create confusion for companies. Reimbursement often lags behind technology, creating uncertainty about whether new tests will be covered by insurance and limiting patient access. Post-market rules, event reporting, and quality programs add compliance and cost burdens. Training and certification needs for skilled workers create workforce shortages, especially in areas with fewer resources, slowing the adoption of NAAT services.
Nucleic Acid Amplification Testing (NAAT) Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Nucleic Acid Amplification Testing (NAAT) Market |
| Market Size in 2024 | USD 9.14 Billion |
| Market Forecast in 2034 | USD 28.90 Billion |
| Growth Rate | CAGR of 12.20% |
| Number of Pages | 213 |
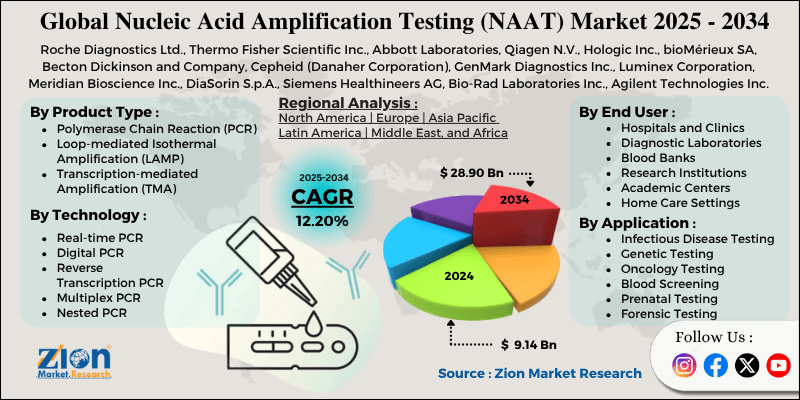
| Key Companies Covered | Roche Diagnostics Ltd., Thermo Fisher Scientific Inc., Abbott Laboratories, Qiagen N.V., Hologic Inc., bioMérieux SA, Becton Dickinson and Company, Cepheid (Danaher Corporation), GenMark Diagnostics Inc., Luminex Corporation, Meridian Bioscience Inc., DiaSorin S.p.A., Siemens Healthineers AG, Bio-Rad Laboratories Inc., Agilent Technologies Inc., and others. |
| Segments Covered | By Product Type, By Technology, By Application, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Nucleic Acid Amplification Testing (NAAT) Market: Segmentation
The global nucleic acid amplification testing market is segmented based on product type, technology, application, end-user, and region.
Based on product type, the global nucleic acid amplification testing industry is divided into polymerase chain reaction (PCR), loop-mediated isothermal amplification (LAMP), transcription-mediated amplification (TMA), strand displacement amplification (SDA), and nucleic acid sequence-based amplification (NASBA). Polymerase chain reaction (PCR) leads the market due to its proven reliability, extensive clinical validation, and widespread familiarity among laboratory professionals worldwide.
Based on technology, the industry is segregated into real-time PCR, digital PCR, reverse transcription PCR, multiplex PCR, nested PCR, and others. Real-time PCR leads the market due to its rapid delivery of results, quantitative capabilities, and reduced risk of contamination compared to traditional gel electrophoresis-based detection methods.
Based on application, the global nucleic acid amplification testing market is classified into infectious disease testing, genetic testing, oncology testing, blood screening, prenatal testing, and forensic testing. Infectious disease testing is expected to lead the market during the forecast period due to its high testing volumes, urgent clinical needs, and continuous emergence of new pathogen threats requiring molecular identification.
Based on end-user, the global market is segmented into hospitals and clinics, diagnostic laboratories, blood banks, research institutions, academic centers, and home care settings. Hospitals and clinics hold the largest market share due to their diverse patient populations, comprehensive testing requirements, and increasing adoption of point-of-care molecular diagnostic capabilities.
Nucleic Acid Amplification Testing (NAAT) Market: Regional Analysis
What factors are contributing to North America's leadership in the global nucleic acid amplification testing market?
North America leads the global nucleic acid amplification testing market because it has advanced healthcare systems, large investments in research and development, and quick adoption of new molecular diagnostic tools. The United States accounts for approximately 40% of global NAAT use, supported by large hospital networks, specialized diagnostic laboratories, and robust public health monitoring programs. The region benefits from leading biotech companies, top research institutions, and strong government support for precision medicine. U.S. manufacturers are creating next-generation sequencing systems, automated sample preparation tools, and AI-based result interpretation platforms. The presence of big pharmaceutical companies and clinical research organizations increases demand for companion diagnostic testing and drug development.
Widespread insurance coverage and good reimbursement policies help expand access to molecular diagnostics. The region’s clear regulatory system supports innovation while ensuring safety and effectiveness. Strong partnerships between universities, hospitals, and industry enable research discoveries to be quickly translated into real-world clinical applications. These combined factors give North America a strong advantage in global NAAT adoption. The high level of healthcare spending also makes new technologies more accessible and easier to use in practice. Continued innovation will keep the region ahead of other markets in this sector.
Europe is showing robust growth.
Europe is experiencing strong growth in the nucleic acid amplification testing market, as many countries invest in healthcare modernization and expand molecular diagnostic use across different medical settings. European Union member states are building national genetic testing plans and precision medicine programs that depend on NAAT for patient selection and treatment planning. The region’s focus on preventive healthcare and early diagnosis is increasing demand for sensitive molecular tests that detect conditions before symptoms appears.
European companies are working on new sample preparation methods, isothermal amplification techniques, and portable devices that solve regional healthcare challenges. Cross-country collaborations and research projects are pushing NAAT development forward by sharing knowledge and resources.
Efforts to control antibiotic resistance and improve infection control are also driving the use of fast pathogen testing and resistance detection tools. Medical tourism and cross-border healthcare policies are helping to standardize testing and open larger markets for diagnostic providers. These trends are making Europe one of the fastest-growing regions for NAAT adoption. Government support and patient awareness are creating more opportunities for molecular testing.
Recent Market Developments:
- In May 2025, SynOligo Biotechnologies partnered with Lumiphore to launch a luminescent lanthanide-based oligo probe (Lumi804-Eu) for NAAT assays, improving sensitivity, reducing background noise, and simplifying assay workflows.
Nucleic Acid Amplification Testing (NAAT) Market: Competitive Analysis
The leading players in the global nucleic acid amplification testing market are:
- Roche Diagnostics Ltd.
- Thermo Fisher Scientific Inc.
- Abbott Laboratories
- Qiagen N.V.
- Hologic Inc.
- bioMérieux SA
- Becton Dickinson and Company
- Cepheid (Danaher Corporation)
- GenMark Diagnostics Inc.
- Luminex Corporation
- Meridian Bioscience Inc.
- DiaSorin S.p.A.
- Siemens Healthineers AG
- Bio-Rad Laboratories Inc.
- Agilent Technologies Inc.
The global nucleic acid amplification testing market is segmented as follows:
By Product Type
- Polymerase Chain Reaction (PCR)
- Loop-mediated Isothermal Amplification (LAMP)
- Transcription-mediated Amplification (TMA)
- Strand Displacement Amplification (SDA)
- Nucleic Acid Sequence-based Amplification (NASBA)
By Technology
- Real-time PCR
- Digital PCR
- Reverse Transcription PCR
- Multiplex PCR
- Nested PCR
- Others
By Application
- Infectious Disease Testing
- Genetic Testing
- Oncology Testing
- Blood Screening
- Prenatal Testing
- Forensic Testing
By End User
- Hospitals and Clinics
- Diagnostic Laboratories
- Blood Banks
- Research Institutions
- Academic Centers
- Home Care Settings
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Nucleic Acid Amplification Testing (NAAT) is a molecular diagnostic method used to amplify and detect specific DNA or RNA sequences for identifying pathogens, genetic changes, and cellular abnormalities with high precision and sensitivity.
The global nucleic acid amplification testing market is projected to grow due to increasing prevalence of infectious diseases, rising demand for rapid diagnostic solutions, and growing emphasis on precision medicine and personalized healthcare.
According to a study, the global nucleic acid amplification testing market size was worth around USD 9.14 billion in 2024 and is predicted to grow to around USD 28.90 billion by 2034.
The CAGR value of the nucleic acid amplification testing market is expected to be around 12.20% during 2025-2034.
North America is expected to lead the global nucleic acid amplification testing market during the forecast period.
The major players profiled in the global nucleic acid amplification testing market include Roche Diagnostics Ltd., Thermo Fisher Scientific Inc., Abbott Laboratories, Qiagen N.V., Hologic Inc., bioMérieux SA, Becton Dickinson and Company, Cepheid (Danaher Corporation), GenMark Diagnostics Inc., Luminex Corporation, Meridian Bioscience Inc., DiaSorin S.p.A., Siemens Healthineers AG, Bio-Rad Laboratories Inc., and Agilent Technologies Inc.
The report examines key aspects of the nucleic acid amplification testing market, including a detailed analysis of existing growth factors and restraints, as well as an examination of future growth opportunities and challenges that will impact the market.
The nucleic acid amplification testing market is being shaped by rapid technological advancements, including automation, digital PCR, isothermal amplification, and artificial intelligence, which make tests faster, more accurate, and easier to use outside of large laboratories. These innovations are expanding adoption in hospitals, clinics, and remote settings worldwide.
The nucleic acid amplification testing market is witnessing leading companies adopt strategies such as new product launches, partnerships, mergers and acquisitions, and geographic expansion to broaden test menus, improve turnaround times, and extend point-of-care availability.
The nucleic acid amplification testing market is led by the hospital and clinic segment, which holds the largest share due to high testing volumes, widespread laboratory infrastructure, and adoption of point-of-care diagnostic systems, followed by diagnostic laboratories for high-throughput applications.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed