Global Nucleic Acid Testing Market Size, Share, Growth Analysis Report - Forecast 2034

Nucleic Acid Testing Market By Indication (Cancer, Infectious Diseases, Personalized Medicine, Forensic Testing, and Others), By Technology (Polymerase Chain Reaction (PCR), Transcription-Mediated Amplification (TMA), Next-Generation Sequencing (NGS), Others), By Application (Infectious Diseases, Cancer, Genetic Disorders, Blood Screening, Others), By End-user (Hospitals, Diagnostic Laboratories, Research Institutes, Blood Banks), By Product Type (Reagents & Consumables, Instruments, and Software & Services), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 8.19 Billion | USD 31.33 Billion | 13.1% | 2024 |
Nucleic Acid Testing Market: Industry Perspective
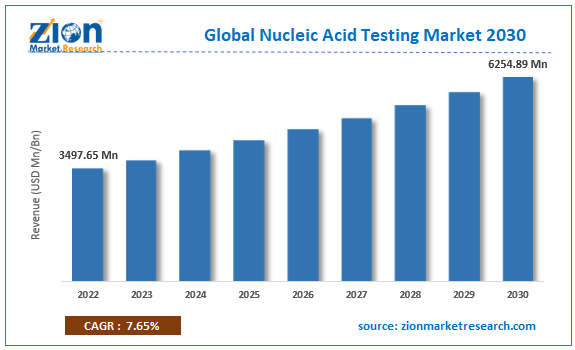
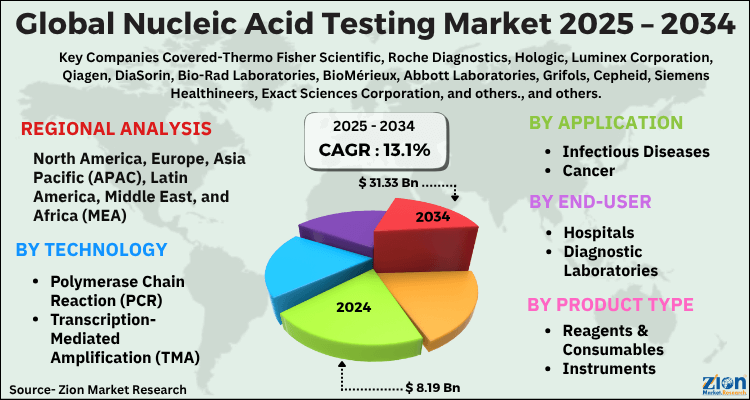
The global nucleic acid testing market size was worth around USD 8.19 Billion in 2024 and is predicted to grow to around USD 31.33 Billion by 2034 with a compound annual growth rate (CAGR) of roughly 13.1% between 2025 and 2034. The report analyzes the global nucleic acid testing market's drivers, restraints, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the nucleic acid testing industry.
Nucleic Acid Testing Market: Overview
Nucleic acid testing (NAT) is a medical and research technique used for detecting nucleic acid sequences for further analysis and detection of particular species or subspecies of an organism. It is used for determining the presence of bacteria or viruses in a given sample acting as pathogens in tissue, blood, urine, and others. These testing methods differ from other methods since NATs depend on genetic components of the organisms such as ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) rather than the presence of antibodies and antigens. Nucleic acid testing is preferred in certain situations because this method allows rapid detection of a certain disease since the development of antigens or antibodies may take some time resulting in delayed detection. In almost all nucleic acid testing procedures, an amplification method is used that helps in increasing the genetic material. This step is required because certain genetic components can be extremely scarce and difficult to detect. In such conditions, NATs are referred to as nucleic acid amplification tests (NAATs) and methods such as strand displacement assay (SDA), polymerase chain reaction (PCR), and transcription-mediated assay (TMA). The nucleic acid testing market is expected to grow at a steady pace during the forecast period.
Key Insights
- As per the analysis shared by our research analyst, the global nucleic acid testing market is estimated to grow annually at a CAGR of around 13.1% over the forecast period (2025-2034).
- Regarding revenue, the global nucleic acid testing market size was valued at around USD 8.19 Billion in 2024 and is projected to reach USD 31.33 Billion by 2034.
- The nucleic acid testing market is projected to grow at a significant rate due to Rising demand for accurate infectious disease diagnosis and genetic testing fuels growth. Advancements in molecular diagnostics and pandemic preparedness support adoption.
- Based on indication, the infectious diseases segment is expected to lead the global market.
- Based on technology, the polymerase chain reaction (PCR) segment is expected to lead the global market.
- On the basis of application, the infectious diseases segment is growing at a high rate and will continue to dominate the global market.
- Based on the end-user, the hospitals segment is projected to swipe the largest market share.
- By product type, the reagents & consumables segment is expected to dominate the global market.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Nucleic Acid Testing Market: Growth Drivers
Growing number of HIV cases to create higher market growth
The global nucleic acid testing market is expected to grow owing to the increasing number of HIV cases across the globe. NATs are used extensively for detecting the presence of RNA or DNA corresponding to the human immunodeficiency virus (HIV). These viruses are known to attach cells present in human bodies that are responsible for fighting infection. Contracting the HIV means that the patient is more vulnerable to other diseases and infections. HIV can spread through coming in contact with specific body fluids of an HIV-positive patient. The most common reason for the growing spread of HIV conditions is unprotected intercourse or sharing an injection between multiple people. In case HIV is left untreated, it can result in (acquired immunodeficiency syndrome (AIDS). It is one of the medical conditions in the world that does not have any cure. Although there have significant strides made in the medical care of patients living with AIDS, the condition can severely impact the quality of life. As per statistics published by the World Health Organization (WHO), around 39 million across the globe will be living with HIV by the end of 2022. Factors such as increased awareness rate about the importance of frequent testing especially among sexually active people and higher availability of NAT kits have resulted in extensive market growth in the last few years.
COVID-19 was a triggering point for momentous growth in NAT procedures across the globe
Since 2022, nucleic acid testing procedures have gained extreme momentum. These tests have been extremely helpful in the early detection of the COVID-19 virus across the globe driving global market growth. The most popular variant during COVID-19 was PCR-based nucleic acid testing because these procedures provided quick and accurate results. The momentum has continued since then. For instance, in May 2023, the National Institute of Virology (NIV), Pune, India launched the first domestically-produced multiplex single-tube real-time RT-PCR test. It is a highly accurate nucleic acid testing method.
Nucleic Acid Testing Market: Restraints
Several technical limitations to restrict market growth
The nucleic acid testing industry players face several technical limitations when dealing with nucleic acid testing. For instance, the testing method is not beneficial in obtaining quantitative results in the case of large-scale clinical samples. The tests are extremely difficult to use for susceptibility testing. In addition to this, advanced PCR NATs can be expensive due to the complex nature of the testing procedure. While the test results are highly accurate, conducting advanced nucleic acid tests demands the use of dedicated infrastructure equipped with all essential systems and technologies.
Nucleic Acid Testing Market: Opportunities
Increasing launch of new NAT processes and rising research in associated technology to deliver excellent expansion possibilities
The global nucleic acid testing market growth trend appears promising due to the increasing number of recent advancements in nucleic acid testing procedures, kits, and supporting systems. For instance, in March 2021, Promega Corporation launched XpressAmp™ Direct Amplification Reagents. This development will allow end-users to prepare samples without the need to extract RNA. It helped laboratories dealing with COVID-19 testing eliminate one of the most prominent bottlenecks in testing procedures. The laboratories could directly move to the PCR amplification process. Furthermore, the increasing use of NATs in emerging economies along with rising investments in the regional healthcare infrastructure. In September 2021, Thermo Fisher Scientific announced that it will start manufacturing nucleic acid testing kits in India as researchers view the tools with applications beyond COVID-19 testing. The test kits developed by Thermo Fisher can analyze up to 96 samples within 30 minutes. On the other hand, in April 2022, Beijing city in China witnessed the launch of massive nucleic acid testing for nearly 90% of its residents as COVID-19 infections continued to be reported in the dense city.
Nucleic Acid Testing Market: Challenges
Lack of infrastructure and quality management to challenge market expansion
The global nucleic acid testing market growth is expected to be challenged due to the lack of infrastructure that aids manufacturing and application of NATs in addition to the issues faced in managing the quality of nucleic acid testing procedures. Furthermore, managing the vast scale of data generated during NAT procedures adds to growth restraints.
Nucleic Acid Testing Market: Segmentation
The global nucleic acid testing market is segmented based on indication, technology, application, end-user, product type, and region.
Based on indication, the global market segments are cancer, infectious diseases, personalized medicine, forensic testing, and others. In 2024, the highest demand was observed in the infectious diseases segment since nucleic acid testing is most significantly used for the rapid detection of contagious diseases that spread quickly from one person to another. The segmental growth in 2024 was a result of increased use of RT-PCR tests during COVID-19. As per research, the current best-in-class assays are known to showcase a limit of detection (LoD) of ~100 copies of viral RNA per milliliter of transport media.
Based on technology, the global nucleic acid testing market is divided into Polymerase Chain Reaction (PCR), Transcription-Mediated Amplification (TMA), Next-Generation Sequencing (NGS), Others.
On the basis of application, the global nucleic acid testing market is bifurcated into Infectious Diseases, Cancer, Genetic Disorders, Blood Screening, Others.
By End-user, the global nucleic acid testing market is split into hospitals, diagnostic laboratories, research institutes, blood banks.
In terms of Product Type, the global nucleic acid testing market is categorized into Reagents & Consumables, Instruments, Software & Services.
Nucleic Acid Testing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Nucleic Acid Testing Market |
| Market Size in 2024 | USD 8.19 Billion |
| Market Forecast in 2034 | USD 31.33 Billion |
| Growth Rate | CAGR of 13.1% |
| Number of Pages | 231 |
| Key Companies Covered | Thermo Fisher Scientific, Roche Diagnostics, Hologic, Luminex Corporation, Qiagen, DiaSorin, Bio-Rad Laboratories, BioMérieux, Abbott Laboratories, Grifols, Cepheid, Siemens Healthineers, Exact Sciences Corporation, and others., and others. |
| Segments Covered | By Technology, By Application, By End-user, By Product Type, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Nucleic Acid Testing Market: Regional Analysis
North America to witness the highest growth rate during the coming period
The global nucleic acid testing market is expected to be led by North America during the forecast period. The US will act as the major regional shareholder driven by the presence of an evolving and globally prominent medical and drug research infrastructure. The US is home to some of the largest pharmaceutical giants constantly working toward developing novel testing methods to detect infectious diseases and develop efficient treatments. Furthermore, the US is highly popular for running large-scale healthcare programs for the medical safety of its population. In 2018, the University of Glasgow-led project was awarded USD 1.85 million to conduct rapid testing in rural and remote locations. The funds were also used for the development of efficient tests for new parasitic diseases. Moreover, the region has a rich ecosystem that supports the development of personalized medicine. As per research, Pfizer is predicted to lead the way for customized medicines in the coming years. In July 2023, Pfizer announced that it would invest USD 100 million in association with venture firm Flagship Pioneering for the development of 10 novel potential drugs including infectious diseases.
Nucleic Acid Testing Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the nucleic acid testing market on a global and regional basis.
The global nucleic acid testing market is dominated by players like:
- Thermo Fisher Scientific
- Roche Diagnostics
- Hologic
- Luminex Corporation
- Qiagen
- DiaSorin
- Bio-Rad Laboratories
- BioMérieux
- Abbott Laboratories
- Grifols
- Cepheid
- Siemens Healthineers
- Exact Sciences Corporation
The global nucleic acid testing market is segmented as follows;
By Indication
- Cancer
- Infectious Diseases
- Personalized Medicine
- Forensic Testing
By Technology
- Polymerase Chain Reaction (PCR)
- Transcription-Mediated Amplification (TMA)
- Next-Generation Sequencing (NGS)
- Others
By Application
- Infectious Diseases
- Cancer
- Genetic Disorders
- Blood Screening
- Others
By End-user
- Hospitals
- Diagnostic Laboratories
- Research Institutes
- Blood Banks
By Product Type
- Reagents & Consumables
- Instruments
- Software & Services
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Nucleic acid testing (NAT) is a medical and research technique used for detecting nucleic acid sequences for further analysis and detection of particular species or subspecies of an organism.
The global nucleic acid testing market is expected to grow due to Rising demand for accurate infectious disease diagnosis and genetic testing fuels growth. Advancements in molecular diagnostics and pandemic preparedness support adoption.
According to a study, the global nucleic acid testing market size was worth around USD 8.19 Billion in 2024 and is expected to reach USD 31.33 Billion by 2034.
The global nucleic acid testing market is expected to grow at a CAGR of 13.1% during the forecast period.
North America is expected to dominate the nucleic acid testing market over the forecast period.
Leading players in the global nucleic acid testing market include Thermo Fisher Scientific, Roche Diagnostics, Hologic, Luminex Corporation, Qiagen, DiaSorin, Bio-Rad Laboratories, BioMérieux, Abbott Laboratories, Grifols, Cepheid, Siemens Healthineers, Exact Sciences Corporation, and others., among others.
The report explores crucial aspects of the nucleic acid testing market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed