Non Invasive Brain Trauma Monitoring Device Market Size, Share, Trends, Growth 2034

Non Invasive Brain Trauma Monitoring Device Market By Type (Noninvasive Intracranial Pressure Monitor, Noninvasive Cerebral Edema Dynamic Monitor, and Others), By Product (Consumables, Electrodes, Sensors, Fiber Optic Cables, Monitoring Devices, Computerized Tomography (CT) Scanners, Intracranial Pressure Monitors, Positron Emission Tomography (PET) Scanners, Electroencephalogram (EEG), Magnetoencephalogram (MEG), and Others), By Application (Cardiology, Urology and Nephrology, Oncology, Gastroenterology, and Others), By End User (Hospitals and Neurological Centers), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 15.4 Billion | USD 35.4 Billion | 8.7% | 2024 |
Non Invasive Brain Trauma Monitoring Device Industry Perspective:
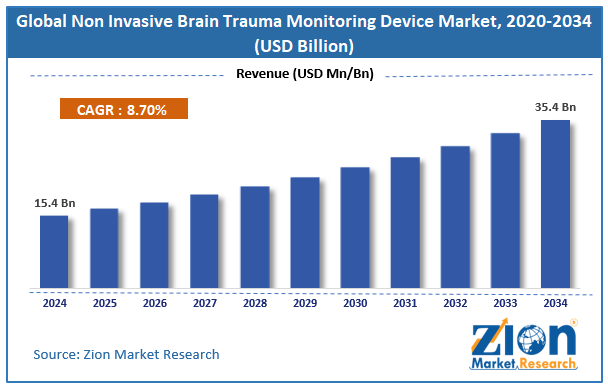
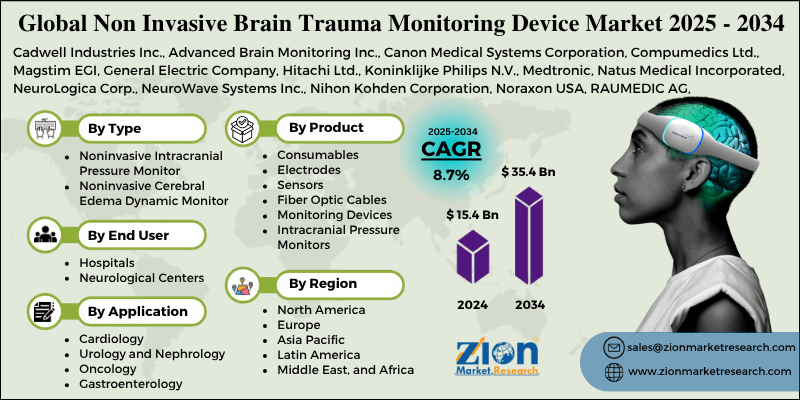
The global non invasive brain trauma monitoring device market size was worth around USD 15.4 billion in 2024 and is predicted to grow to around USD 35.4 billion by 2034, with a compound annual growth rate (CAGR) of roughly 8.7% between 2025 and 2034.
Non Invasive Brain Trauma Monitoring Device Market: Overview
A non invasive brain trauma monitoring device is defined as a device that checks the brain health without going through the skull or brain tissue. These devices enable neurologists and other clinicians to assess the severity of the brain injury, monitor its recovery, and prevent further damage.
They use multiple equipment to keep an eye on blood flow, oxygen levels, and brain electrical activity, which makes them less invasive. The non invasive brain trauma monitoring device industry expansion is mainly driven by several variables such as the growing incidence of TBIs, the shift towards non-invasive diagnostics, innovation in technology, and growing healthcare infrastructure and funding.
Key Insights
- As per the analysis shared by our research analyst, the global Non Invasive Brain Trauma Monitoring Device market is estimated to grow annually at a CAGR of around 8.7% over the forecast period (2025-2034).
- In terms of revenue, the global Non Invasive Brain Trauma Monitoring Device market size was valued at around USD 15.4 billion in 2024 and is projected to reach USD 35.4 billion by 2034.
- The growing elderly population with neurological disorders is expected to drive the non invasive brain trauma monitoring device market over the forecast period.
- Based on the type, the noninvasive intracranial pressure monitor segment is expected to hold the largest market share over the forecast period.
- Based on the product, the monitoring devices segment is expected to dominate the market expansion over the projected period.
- Based on the application, the cardiology segment is expected to dominate the market expansion over the projected period.
- Based on the end-user, the hospitals segment is expected to dominate the market expansion over the projected period.
- Based on region, North America is expected to dominate the market during the forecast period.
Non Invasive Brain Trauma Monitoring Device Market: Growth Drivers
Growing number of traumatic brain injuries across the globe drives market growth
Among all trauma-related injuries, brain injury (TBI), also known as the silent epidemic, is the leading cause of death and disability worldwide and continues to be an increasing public health concern. According to the European Brain Injury Consortium, around 50 million people globally have a TBI each year, with 80% of those cases occurring in developing nations.
Similarly, as per the data published by the Centre for Neuro Skills, above 5.3 million Americans are living today with TBI-related injuries. Some of the major causes of traumatic brain injury (TBI) are road traffic collisions, falls, and violence. Consequently, rising rates of traumatic brain injuries (TBIs) worldwide are driving the invasive brain trauma monitoring device market expansion.
Non Invasive Brain Trauma Monitoring Device Market: Restraints
The high cost of advanced devices hinders market growth
The expensive cost of non invasive brain trauma monitoring devices is one of the biggest impediments to their wider adoption, which impacts both end users and healthcare practitioners, particularly in developing nations. These devices use advanced technology, which includes transcranial Doppler (TCD), EEG sensors, near-infrared spectroscopy (NIRS), and artificial intelligence (AI) algorithms. These integrations are very costly, consequently raising their cost.
Moreover, significant expenditures are also made in clinical trials and research to guarantee precision, security, and adherence. Furthermore, acquiring FDA, CE, or other clearances for medical devices entails costly procedures. Therefore, the non invasive brain trauma monitoring devices market is severely constrained by the above-described problem.
Non Invasive Brain Trauma Monitoring Device Market: Opportunities
Rising acquisition among the key market players offers a lucrative opportunity for market growth
The growing acquisition among the key market players is expected to flourish the non invasive brain trauma monitoring device industry during the forecast period.
For instance, in June 2023, Spiegelberg GmbH & Co. KG (Spiegelberg), an established medical device producer and supplier of highly specialized equipment and supplies for neurosurgery, was acquired by Luciole Medical AG, a Swiss medical technology business that specializes in brain monitoring, from SHS Capital.
To maximize their capabilities and become a global leader in the provision of cutting-edge next-generation brain monitoring devices, the companies will integrate their product suites, manufacturing facilities, and distribution networks after the acquisition.
Non Invasive Brain Trauma Monitoring Device Market: Challenges
Shortage of skilled labor poses a major challenge to market expansion
Non-invasive brain trauma monitoring devices are introducing new cybersecurity vulnerabilities and data privacy issues as they use digital health technologies more and more, such as cloud connectivity, remote monitoring, AI analytics, and wireless data transmission. These problems have grown to be significant market barriers, particularly as governments and healthcare systems around the world place more importance on data protection laws like GDPR (EU), HIPAA (US), and others.
For instance, cybersecurity experts identified some digital EEG systems in 2023 for having inadequate encryption on cloud dashboards. Between 2022 and 2024, networked medical devices have been the target of an increasing number of healthcare ransomware attacks, particularly in intensive care units and emergency rooms. Therefore, the aforementioned facts pose a major challenge to the non invasive brain trauma monitoring device sector.
Non Invasive Brain Trauma Monitoring Device Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Non Invasive Brain Trauma Monitoring Device Market |
| Market Size in 2024 | USD 15.4 Billion |
| Market Forecast in 2034 | USD 35.4 Billion |
| Growth Rate | CAGR of 8.7% |
| Number of Pages | 213 |
| Key Companies Covered | Cadwell Industries Inc., Advanced Brain Monitoring Inc., Canon Medical Systems Corporation, Compumedics Ltd., Magstim EGI, General Electric Company, Hitachi Ltd., Koninklijke Philips N.V., Medtronic, Natus Medical Incorporated, NeuroLogica Corp., NeuroWave Systems Inc., Nihon Kohden Corporation, Noraxon USA, RAUMEDIC AG, Sense Neuro, Siemens, Sophysa, Spiegelberg GmbH & Co. KG, and others. |
| Segments Covered | By Type, By Product, By Application, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Non Invasive Brain Trauma Monitoring Device Market: Segmentation
The global non invasive brain trauma monitoring device industry is segmented based on type, product, application, end-user, and region.
Based on the type, the global market is bifurcated into noninvasive intracranial pressure monitor, noninvasive cerebral edema dynamic monitor, and others. The noninvasive intracranial pressure monitor segment is expected to hold the largest market share over the forecast period. A noninvasive intracranial pressure (ICP) monitor is a medical device that measures intracranial pressure (ICP) without the need for a catheter or probe to be surgically inserted. These are particularly utilized in cases of stroke, hydrocephalus, traumatic brain injury (TBI), and other neurological conditions. The segment expansion is driven by the rising occurrence of stroke and TBI.
Based on the product, the global non invasive brain trauma monitoring device industry is bifurcated into Consumables, Electrodes, Sensors, Fiber Optic Cables, Monitoring Devices, Computerized Tomography (CT) Scanners, Intracranial Pressure Monitors, Positron Emission Tomography (PET) Scanners, Electroencephalogram (EEG), Magnetoencephalogram (MEG) and Others.
The Monitoring Devices segment is expected to dominate the market over the projected period. The increasing need for non-invasive methods of treating brain illnesses is the reason for the sector's increase. Intracranial pressure monitors, MRI and CT scanners, EEG and MEG devices, and other devices are further subdivided into monitoring devices. These gadgets assist in generating revenue as their cost is significantly higher than that of consumables. As medical facilities strive to have the newest monitoring equipment for treatments, the market is also expected to expand in the healthcare industry.
Based on the application, the global non invasive brain trauma monitoring device market is bifurcated into cardiology, urology and nephrology, oncology, gastroenterology, and others. The cardiology segment is expected to capture the largest market share over the forecast period. The increasing number of cardiology patients across the globe is expected to drive the industry expansion.
Based on the end user, the global market is bifurcated into hospitals and neurological centers. The hospitals segment is expected to hold the largest market share over the projected period due to the significant rise in brain surgeries conducted in hospitals worldwide.
Non Invasive Brain Trauma Monitoring Device Market: Regional Analysis
North America dominates the market over the projected period
North America is expected to dominate the global non invasive brain trauma monitoring device market during the anticipated period. This can be attributed to the increasing use of non-invasive brain trauma monitoring tools and the presence of large medical institutions that have invested significantly in the development of medical technology. This region's market has grown as a result of the growing number of elderly people with neurological disorders.
However, the Asia Pacific is expected to grow at the highest CAGR during the projected period, owing to the increasing cases of neurological disorders and the increasing aging population.
Non Invasive Brain Trauma Monitoring Device Market: Competitive Analysis
The global non invasive brain trauma monitoring device market is dominated by players like:
- Cadwell Industries Inc.
- Advanced Brain Monitoring Inc.
- Canon Medical Systems Corporation
- Compumedics Ltd.
- Magstim EGI
- General Electric Company
- Hitachi Ltd.
- Koninklijke Philips N.V.
- Medtronic
- Natus Medical Incorporated
- NeuroLogica Corp.
- NeuroWave Systems Inc.
- Nihon Kohden Corporation
- Noraxon USA
- RAUMEDIC AG
- Sense Neuro
- Siemens
- Sophysa
- Spiegelberg GmbH & Co. KG
The global non invasive brain trauma monitoring device market is segmented as follows:
By Type
- Noninvasive Intracranial Pressure Monitor
- Noninvasive Cerebral Edema Dynamic Monitor
- Others
By Product
- Consumables
- Electrodes
- Sensors
- Fiber Optic Cables
- Monitoring Devices
- Computerized Tomography (CT) Scanners
- Intracranial Pressure Monitors
- Positron Emission Tomography (PET) Scanners
- Electroencephalogram (EEG)
- Magnetoencephalogram (MEG)
- Others
By Application
- Cardiology
- Urology and Nephrology
- Oncology
- Gastroenterology
- Others
By End User
- Hospitals
- Neurological Centers
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
A non invasive brain trauma monitoring device is defined as a device that checks the brain health without going through the skull or brain tissue. These devices enable neurologists and other clinicians to assess the severity of the brain injury, monitor its recovery, and prevent further damage.
The non invasive brain trauma monitoring device market expansion is mainly driven by several variables such as the growing incidence of TBIs, the shift towards non-invasive diagnostics, innovation in technology, and growing healthcare infrastructure and funding.
According to the report, the global non invasive brain trauma monitoring device market size was worth around USD 15.4 billion in 2024 and is predicted to grow to around USD 35.4 billion by 2034.
The global non invasive brain trauma monitoring device market is expected to grow at a CAGR of 8.7% during the forecast period.
The global non invasive brain trauma monitoring device market growth is expected to be driven by North America. It is currently the world’s highest revenue-generating market due to the developed healthcare infrastructure and the presence of major key players in the area.
The global non invasive brain trauma monitoring device market is dominated by players like Cadwell Industries, Inc., Advanced Brain Monitoring, Inc., Canon Medical Systems Corporation, Compumedics Ltd., Magstim EGI, General Electric Company, Hitachi, Ltd., Koninklijke Philips N.V., Medtronic, Natus Medical Incorporated, NeuroLogica Corp., NeuroWave Systems, Inc., Nihon Kohden Corporation, Noraxon USA, RAUMEDIC AG, Sense Neuro, Siemens, Sophysa, and Spiegelberg GmbH & Co. KG, among others.
The non invasive brain trauma monitoring device market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTLE analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five forces analysis, and value chain analysis.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed