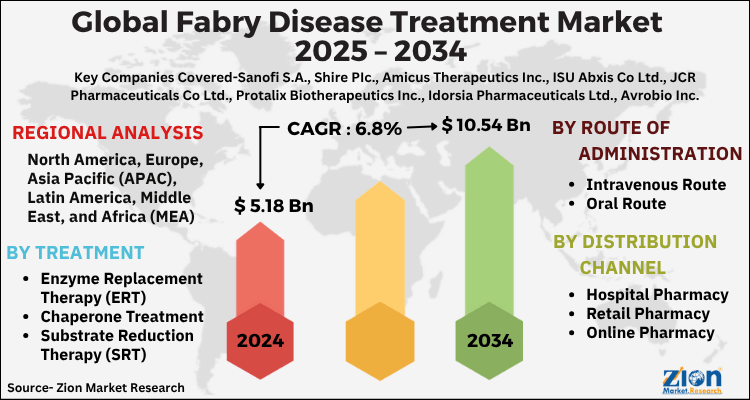Global Fabry Disease Treatment Market Size, Share, Growth Analysis Report - Forecast 2034

Fabry Disease Treatment Market By Treatment (Enzyme Replacement Therapy (ERT), Chaperone Treatment, Substrate Reduction Therapy (SRT), Others), By Route of Administration (Intravenous Route, Oral Route), By Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 5.18 Billion | USD 10.54 Billion | 6.8% | 2024 |
Global Fabry Disease Treatment Market: Industry Perspective
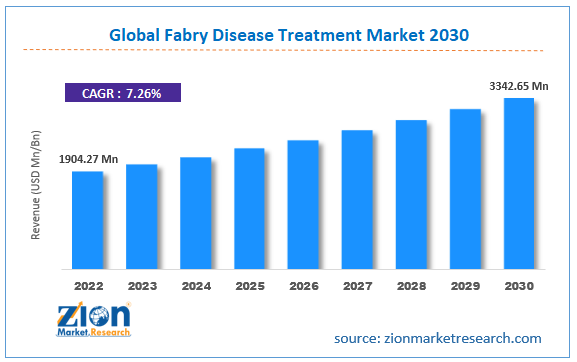
The global fabry disease treatment market size was worth around USD 5.18 Billion in 2024 and is predicted to grow to around USD 10.54 Billion by 2034 with a compound annual growth rate (CAGR) of roughly 6.8% between 2025 and 2034. The report analyzes the global fabry disease treatment market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the fabry disease treatment industry.
Global Fabry Disease Treatment Market: Overview
Fabry disease is a genetic disease passed on from the parents to children and is caused due to the buildup of globotriaosylceramide, a type of fat. The disease starts showing signs and symptoms at the beginning of childhood and some of the most commonly observed symptoms are frequent episodes of pain especially concentrated in body areas such as feet & hands, clusters of dark, small, red spots on the skin also known as angiokeratomas, cloudiness in front of the eyes, reduced ability to sweat or (hypohidrosis), and ringing in the ears. In addition to this, patients may also complain of hearing loss and problems with the gastrointestinal system. However, the signs are not limited to only these conditions as they may vary from one patient to another with specific groups showing other signs. If left untreated or undiagnosed, Fabry disease can also lead to life-threatening complications such as stroke, heart failure, and progressive kidney failure. In some patients, the condition may show milder symptoms that may appear in later stages of adulthood and may be concentrated only in areas including blood vessels in the brain, kidney, or heart.
Global Fabry Disease Treatment Market: Growth Drivers
Increasing prevalence of Fabry disease to create higher demand for treatment
The global Fabry disease treatment market is projected to grow owing to the increasing prevalence of the condition. As per research analysis, the condition affects 1 in 1000 to 9000 people. The rising population rate increases the chances of the condition spreading to newborns since it is a hereditary disorder. Recent observations conclude that the chances of a person developing milder symptoms with age are more common than contracting the severe form. Moreover, people of all ethnicities and genders are vulnerable to Fabry disease leading to the condition affecting a broader group of patients. In addition to this, awareness around Fabry disease is growing rapidly as discussions in the scientific and medical community are on the rise. People have become more understanding of the associated symptoms leading to early diagnosis which plays a crucial role in driving the market revenue further.
Rising innovation in diagnostic technologies to help the industry expand further
Fabry disease is generally considered an under-diago condition and until a few years ago, appropriate diagnostic tools for determining Fabry disease in a patient with complete accuracy were difficult. However, with the ongoing technological advancements in the medical and healthcare industry, efficient and accurate diagnosis is possible. In August 2018, PerkinElmer received US Food and Drug Administration (FDA) approval for the commercial sale of NeoLSD MSMS Kit, a new tool with the capability to detect around 6 lysosomal storage disorders including Fabry disease in newborns. The test can be conducted using only blood samples.
Fabry Disease Treatment Market: Restraints
Lack of cure against Fabry disease to restrict market growth
The global Fabry disease treatment market growth may be restricted since there is no cure for the condition. The treatments available in the healthcare industry can also treat symptoms and alleviate associated pain. As of 2023, the only effective treatment available is enzyme replacement therapy (ERT). However, medical professionals may also recommend treatment such as adjunct therapies along with conventional medical treatment. It may include making changes in lifestyle, eating, and other habits. However, Fabry disease cannot be eliminated. Furthermore, since the treatment is long-lasting the cost is also relatively high thus limiting the number of patients that can undergo the treatment process.
Fabry Disease Treatment Market: Opportunities
Increasing approval of new treatment therapies for commercial application could create higher growth opportunities
The Fabry disease treatment industry players are expected to explore higher growth possibilities owing to the ongoing research in understanding the disease and the symptoms while also investing in developing novel therapies for treatment. Since the condition can impact a large segment of the growing population, more pharmaceutical players and healthcare agencies are working toward creating effective treatment plans along with improved diagnostic tools. In May 2023, the US FDA approved the use of pegunigalsidase alfa. It is an ERT to be used for treating confirmed cases of Fabry disease in adults.
Rising healthcare investment to provide scope for new growth avenues
The growing demand for adequate and quality healthcare has led to an increase in global healthcare expenditure with regional governments and international agencies working toward creating a healthcare ecosystem that is accessible to all. As per the World Health Organization, global health expenditure has reached a mark of USD 9 trillion.
Fabry Disease Treatment Market: Challenges
Lack of supporting healthcare infrastructure to challenge market growth
The Fabry disease treatment industry is likely to be challenged by the lack of supporting healthcare infrastructure especially in developing and underdeveloped countries. Nations with high population rates do not have effective healthcare programs since a large part of the population does not have access to primary medical care. Additionally, since the disease is not as widespread as other life-threatening conditions, a limited number of people are aware of the disease or available treatment. There is a significant gap in the supply and demand for skilled medical professionals who understand the condition thoroughly, further creating barriers against growth.
Key Insights
- As per the analysis shared by our research analyst, the global fabry disease treatment market is estimated to grow annually at a CAGR of around 6.8% over the forecast period (2025-2034).
- Regarding revenue, the global fabry disease treatment market size was valued at around USD 5.18 Billion in 2024 and is projected to reach USD 10.54 Billion by 2034.
- The fabry disease treatment market is projected to grow at a significant rate due to growing disease awareness and diagnosis, advancements in enzyme replacement therapy and emerging gene therapies, rising R&D investments, and favorable regulatory support.
- Based on Treatment, the Enzyme Replacement Therapy (ERT) segment is expected to lead the global market.
- On the basis of Route of Administration, the Intravenous Route segment is growing at a high rate and will continue to dominate the global market.
- Based on the Distribution Channel, the Hospital Pharmacy segment is projected to swipe the largest market share.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Global Fabry Disease Treatment Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Fabry Disease Treatment Market |
| Market Size in 2024 | USD 5.18 Billion |
| Market Forecast in 2034 | USD 10.54 Billion |
| Growth Rate | CAGR of 6.8% |
| Number of Pages | 230 |
| Key Companies Covered | Sanofi S.A., Shire PIc., Amicus Therapeutics Inc., ISU Abxis Co Ltd., JCR Pharmaceuticals Co Ltd., Protalix Biotherapeutics Inc., Idorsia Pharmaceuticals Ltd., Avrobio Inc., Takeda Pharmkceutical Co Ltd., Chiesi Farmaceutici SpA, Freeline Therapeutics Holdings PLC, Yuhan Corp, MOP Therapeutics, and others. |
| Segments Covered | By Treatment, By Route of Administration, By Distribution Channel, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Fabry Disease Treatment Market: Segmentation
The global Fabry disease treatment market is segmented based on Treatment, Route of Administration, Distribution Channel, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2025 to 2034.
Based on treatment, the global Fabry disease treatment market segments are substrate reduction therapy, enzyme replacement therapy, chaperone treatment, and others. In 2024, the highest demand was observed for enzyme replacement therapy since the treatment is accepted across countries including Europe and North America. The rising number of ERT-oriented treatment programs has helped the segment grow further. For instance, using Fabrazyme during ERT helps in restoring alpha-galactosidase A levels thus allowing the body to break down lipids and provide relief from symptoms associated with Fabry disease. The average lifetime expectancy of male patients is around 58 years.
On the basis of Route of Administration, the global fabry disease treatment market is bifurcated into Intravenous Route, Oral Route.
By Distribution Channel, the global fabry disease treatment market is split into Hospital Pharmacy, Retail Pharmacy, Online Pharmacy.
The Regional, this segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America,and the Middle East and Africa.
Global Fabry Disease Treatment Market: Regional Analysis
The Fabry Disease Treatment market exhibits significant regional variation, with North America holding a dominant share due to advanced healthcare infrastructure, higher diagnosis rates, and strong presence of key pharmaceutical players driving research and development of novel therapies. Europe follows closely, supported by increasing awareness, government initiatives for rare diseases, and reimbursement policies facilitating access to high-cost treatments like enzyme replacement therapies and emerging gene therapies. Meanwhile, the Asia-Pacific region is expected to witness the fastest growth, fueled by improving healthcare systems, rising awareness of rare diseases, and expanding patient access to innovative treatments, although challenges like underdiagnosis and limited specialized care persist. Other regions, including Latin America and the Middle East & Africa, are gradually progressing, driven by growing healthcare investments and international collaborations to enhance rare disease management.
Global Fabry Disease Treatment Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the fabry disease treatment market on a global and regional basis.
The global fabry disease treatment market is dominated by players like:
- Sanofi S.A.
- Shire PIc.
- Amicus Therapeutics Inc.
- ISU Abxis Co Ltd.
- JCR Pharmaceuticals Co Ltd.
- Protalix Biotherapeutics Inc.
- Idorsia Pharmaceuticals Ltd.
- Avrobio Inc.
- Takeda Pharmkceutical Co Ltd.
- Chiesi Farmaceutici SpA
- Freeline Therapeutics Holdings PLC
- Yuhan Corp
- MOP Therapeutics
Global Fabry Disease Treatment Market: Segmentation Analysis
The global fabry disease treatment market is segmented as follows;
By Treatment
- Enzyme Replacement Therapy (ERT)
- Chaperone Treatment
- Substrate Reduction Therapy (SRT)
- Others
By Route of Administration
- Intravenous Route
- Oral Route
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
Global Fabry Disease Treatment Market: Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
Fabry disease is a genetic disease passed on from the parents to children and is caused due to the buildup of globotriaosylceramide, a type of fat. The disease starts showing signs and symptoms at the beginning of childhood and some of the most commonly observed symptoms are frequent episodes of pain especially concentrated in body areas such as feet & hands, clusters of dark, small, red spots on the skin also known as angiokeratomas, cloudiness in front of the eyes, reduced ability to sweat or (hypohidrosis), and ringing in the ears. In addition to this, patients may also complain of hearing loss and problems with the gastrointestinal system.
The global fabry disease treatment market is expected to grow due to increasing disease awareness, advancements in enzyme replacement therapies (ERT) and gene therapies, rising healthcare investments, and improved diagnostic technologies.
According to a study, the global fabry disease treatment market size was worth around USD 5.18 Billion in 2024 and is expected to reach USD 10.54 Billion by 2034.
The global fabry disease treatment market is expected to grow at a CAGR of 6.8% during the forecast period.
North America is expected to dominate the fabry disease treatment market over the forecast period.
Leading players in the global fabry disease treatment market include Sanofi S.A., Shire PIc., Amicus Therapeutics Inc., ISU Abxis Co Ltd., JCR Pharmaceuticals Co Ltd., Protalix Biotherapeutics Inc., Idorsia Pharmaceuticals Ltd., Avrobio Inc., Takeda Pharmkceutical Co Ltd., Chiesi Farmaceutici SpA, Freeline Therapeutics Holdings PLC, Yuhan Corp, MOP Therapeutics, among others.
The report explores crucial aspects of the fabry disease treatment market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed