Global E-Clinical Solutions Market Size, Share, Growth Analysis Report - Forecast 2034

E-Clinical Solutions Market By Product (Electronic Data Capture (EDC), Clinical Data Management Systems (CDMS), Clinical Trial Management Systems (CTMS), Randomization and Trial Supply Management (RTSM), eCOA, ePRO, eTMF, Others), By Deployment Model (Web-hosted, Cloud-based, Licensed Enterprise (On-premise)), By Application (Clinical Trials, Clinical Research, Others), By Clinical Trial Phase (Phase I, Phase II, Phase III, Phase IV), By End-user (Pharmaceutical & Biopharmaceutical Companies, Contract Research Organizations (CROs), Academic Research Institutions, Others), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 9.36 Billion | USD 21.90 Billion | 8.87% | 2024 |
E-Clinical Solutions Industry Perspective:
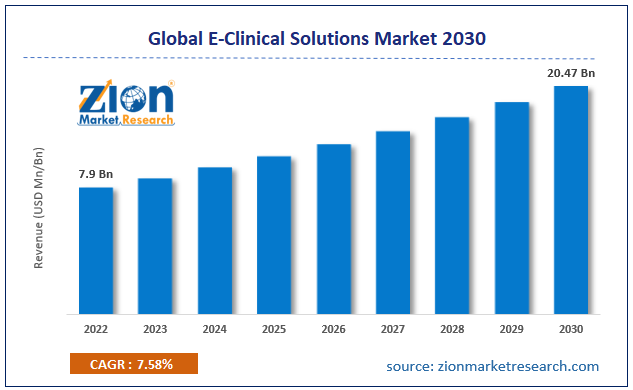
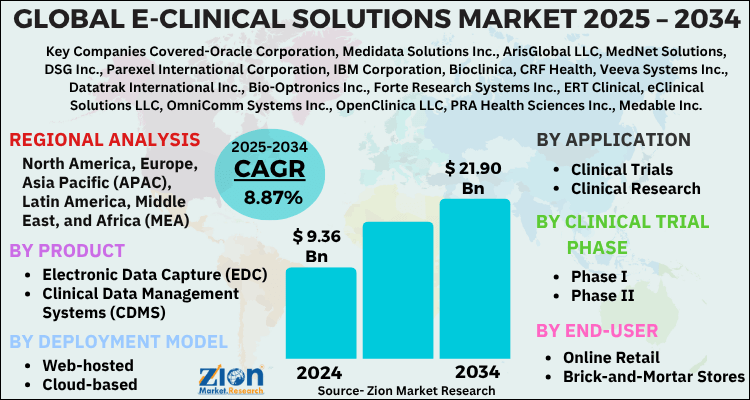
The global e-clinical solutions market size was worth around USD 9.36 Billion in 2024 and is predicted to grow to around USD 21.90 Billion by 2034 with a compound annual growth rate (CAGR) of roughly 8.87% between 2025 and 2034. The report analyzes the global e-clinical solutions market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the e-clinical solutions industry.
The report delves deeper into several crucial aspects of the global E-clinical solutions industry. It includes a detailed discussion of existing growth factors and restraints. Future growth opportunities and challenges that impact the E-clinical solutions market are comprehensively addressed in the report.
E-Clinical Solutions Market: Overview
Clinical research is a sub-segment of medicine. It deals with researching and determining the efficiency of devices, medications, and diagnostic tools that are used in the healthcare industry. Clinical research is one of the largest investments and activities carried out by companies operating in pharmaceutical, healthcare, and medical device manufacturing devices since all of these products have to undergo clinical trials and research.
When the outcome of the trial is positive, the products can be made available for commercial sale once they obtain the necessary approvals from regional agencies that regulate these products. Clinical trials and research are not only cost-intensive but also consumes a lot of time and effort which is a key challenge for the associated market players. E-clinical solutions are a set of technologies that work toward streamlining all the processes involved in clinical trials. It makes use of advanced systems to optimize, by integrating and automating, several aspects associated with clinical research.
Key Insights
- As per the analysis shared by our research analyst, the global e-clinical solutions market is estimated to grow annually at a CAGR of around 8.87% over the forecast period (2025-2034).
- Regarding revenue, the global e-clinical solutions market size was valued at around USD 9.36 Billion in 2024 and is projected to reach USD 21.90 Billion by 2034.
- The e-clinical solutions market is projected to grow at a significant rate due to Need for efficient clinical trial management and regulatory compliance fuels growth. Rising R&D investments and cloud-based tools support adoption.
- Based on Product, the Electronic Data Capture (EDC) segment is expected to lead the global market.
- On the basis of Deployment Model, the Web-hosted segment is growing at a high rate and will continue to dominate the global market.
- Based on the Application, the Clinical Trials segment is projected to swipe the largest market share.
- By Clinical Trial Phase, the Phase I segment is expected to dominate the global market.
- In terms of End-user, the Pharmaceutical & Biopharmaceutical Companies segment is anticipated to command the largest market share.
- Based on region, North America is predicted to dominate the global market during the forecast period.
E-Clinical Solutions Market: Growth Drivers
Growing complexities in drug development procedures to drive market growth
The global E-clinical solutions market is projected to grow due to the increasing complexities in drug development procedures. Regional governments and healthcare regulatory agencies have become strict with protocols surrounding drug approval for commercial purposes. Pharmaceutical companies and medical device makers have adopted an agile development approach where sub-segments such as clinical teams work independently for better output.
As the demand for higher-grade patient care has grown over the years, these companies are under tremendous pressure to ensure that they can keep up with changing consumer expectations. Moreover, drug developers are also looking for measures that allow asset optimization along with risk management.
E-clinical solutions are a means of offering a set of technologies that helps in automating certain processes and helps companies in managing resources more efficiently while saving time. Growing investments in clinical research for all types of medical conditions will contribute to higher adoption of E-clinical solutions. In December 2021, Pfizer inaugurated a state-of-the-art facility in Durham. It is a clinical manufacturing facility worth USD 68.5 million. Several other pharmaceutical companies have invested in building advanced clinical research facilities equipped with superior grad technology.
E-Clinical Solutions Market: Restraints
High implementation cost to restrict market expansion
E-clinical solutions can be costly, especially for companies with limited means and resources. Moreover, the associated cost is not only limited to product purchase but also deals with integration of the new systems in the existing technologies which can take time along with adding to the total cost. Phase IV clinical trials and research can cost anything between USD 50,000 to USD 150 million and smaller companies may be unable to spend more money on additional technologies such as E-clinical solutions. Although there is a scope to leverage the benefit of these solutions using software-as-a-service (SaaS) models, this would mean that drug developers have to rely heavily on third-party services and they will have less control over the systems.
E-Clinical Solutions Market: Opportunities
Increasing competition in the pharmaceutical industry to open growth avenues
The global E-clinical solutions market may come across higher growth opportunities led by the growing internal competition in the pharmaceutical industry across the globe. With changing expectations from drug producers along with the rising entry of new and domestic companies, pharmaceutical companies are currently seeking ways to stay ahead of their competitors.
For instance, in November 2022, CV6 Therapeutics, a leading drug development company, announced its plans to invest £8 toward first-stage clinical trials on CV6-168 which is the company’s first anti-cancer drug. Similarly in October 2022, the University of Texas MD Anderson Cancer Center, and the Focus Fund GP, LLC entered and launched an investment fund focused on advancement in cancer treatment therapies. Such initiatives are not limited to cancer research but can be witnessed for other diseases as well including rare autoimmune conditions and sexually transmitted diseases.
E-Clinical Solutions Market: Challenges
Limited adoption in emerging economies to challenge the market expansion
Since E-clinical solutions demand extensive application of key resources, the adoption rate of the technology has been limited in emerging economies. Although there is significant demand for these products in developed nations, it would be difficult for companies in the E-clinical solutions industry to understand the true performance efficiency and loopholes in the system unless they are adopted on a larger scale. However, budgetary restraints along with a lack of skilled labor to work with E-clinical solutions may add to the existing challenges in the global market.
E-Clinical Solutions Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | E-Clinical Solutions Market |
| Market Size in 2024 | USD 9.36 Billion |
| Market Forecast in 2034 | USD 21.90 Billion |
| Growth Rate | CAGR of 8.87% |
| Number of Pages | PagesNO |
| Key Companies Covered | Oracle Corporation, Medidata Solutions Inc., ArisGlobal LLC, MedNet Solutions, DSG Inc., Parexel International Corporation, IBM Corporation, Bioclinica, CRF Health, Veeva Systems Inc., Datatrak International Inc., Bio-Optronics Inc., Forte Research Systems Inc., ERT Clinical, eClinical Solutions LLC, OmniComm Systems Inc., OpenClinica LLC, PRA Health Sciences Inc., Medable Inc., Merge Healthcare and many more., and others. |
| Segments Covered | By Product, By Deployment Model, By Application, By Clinical Trial Phase, By End-user, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2024 |
| Forecast Year | 2025 to 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
E-Clinical Solutions Market: Segmentation
The global E-clinical solutions market is segmented based on end-user, development phase, delivery mode, product, and region.
Based on end-user, the global market divisions are medical device manufacturers, CROs, hospitals / healthcare providers, pharma & biotech organizations, academic institutes, and others. The highest CAGR was observed in the contract research organizations (CROs) segment in 2022 with a contribution across 36.9% of the market share. During the forecast period, it is likely to grow at a CAGR of 14.1% as many companies are showing an inclination toward effective cost management.
The segmental growth may be driven by the growing demand and adoption of outsourced services which not only reflects in the cost parameters but also across productivity and focus on core competencies attributes. The pharma & biotech organization segment may also register substantial growth as pharma companies are currently witnessing intense competition from local and multinational players.
Based on the development phase, the E-clinical solutions industry is divided into phase IV, phase III, phase II, and phase I. In 2022, around 51.02% of the market share was led by the phase III segment. In this phase, new drugs are compared on parameters such as effectiveness and safety against the existing medication in the market.
Phase III clinical trial is responsible for determining which drug is better against a certain treatment. As per studies, more than 58% of the drugs are unable to pass phase III trials which makes the use of E-clinical solutions in this segment more crucial. As per estimated, phase I segment may grow at a CAGR of 14.81% by 2030.
Based on delivery mode, the global market is divided into licensed enterprises, web-hosted, and others. In 2022, the web-hosted segment dominated industry growth with a market share of over 73%. Several attributes including ease of use along with the requirement of lower investments and access to a dedicated team of professionals are reasons for higher segmental growth. The majority of the web-hosted products offer customization along with showcasing a greater interoperability level. The cloud-based segment may grow at a CAGR of 15.01% driven by the flexibility offered and the availability of real-time data.
Based on product, the E-clinical solutions industry segments are clinical analytics platforms, electronic data capture (EDC), clinical data management system (CDMS), electronic trial master file (eTMF), electronic clinical outcome assessment (eCOA), randomization and trial supply management (RTSM), clinical trial management systems (CTMS), safety solutions, and others.
The CTMS segment accounted for nearly 22.1% of the market share thus acting as the key contributor. During the forecast period, eCOA is projected to become the fastest-growing segment with a CAGR of 15.21% as there is a growing demand for high-quality clinical data. The extensive application in clinician-reported, patient-reported, and observer-reported outcomes could trigger segmental dominance.
E-Clinical Solutions Market: Regional Analysis
North America to control more than 50% of the market share
The global E-clinical solutions market is expected to be led by North America as it may dominate close to 51.1% of the global revenue driven by the increasing research & development along with rising product launches in terms of E-clinical solutions. In recent times, the US government has announced multiple lucrative offers including grants for the players operating in the segment which could help in further growth. In October 2021, the US Food & Drugs Administration (FDA) awarded 11 grants that will be used in clinical trials for the development of medicines for treating rare diseases. The total grant value was around USD 25 million.
Additionally, a growing base of patients diagnosed with life-threatening conditions along with the presence of key players providing exceptionally advanced E-solutions services will help North America in further growth. An example is the November 2022 launch of the Rave companion introduced by Medidata. It is the first scalable solution that automates the entry of electronic health records (EHR) into Rave electronic data capture (EDC). Additionally, in February 2021, NeoGenomics and Parexel, both US-based companies, announced a partnership that will help in improving precision medicine applications in oncology clinical trials.
E-Clinical Solutions Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the e-clinical solutions market on a global and regional basis.
The global e-clinical solutions market is dominated by players like:
- Oracle Corporation
- Medidata Solutions Inc.
- ArisGlobal LLC
- MedNet Solutions
- DSG Inc.
- Parexel International Corporation
- IBM Corporation
- Bioclinica
- CRF Health
- Veeva Systems Inc.
- Datatrak International Inc.
- Bio-Optronics Inc.
- Forte Research Systems Inc.
- ERT Clinical
- eClinical Solutions LLC
- OmniComm Systems Inc.
- OpenClinica LLC
- PRA Health Sciences Inc.
- Medable Inc.
- Merge Healthcare and many more.
The global e-clinical solutions market is segmented as follows;
By Product
- Electronic Data Capture (EDC)
- Clinical Data Management Systems (CDMS)
- Clinical Trial Management Systems (CTMS)
- Randomization and Trial Supply Management (RTSM)
- eCOA
- ePRO
- eTMF
- Others
By Deployment Model
- Web-hosted
- Cloud-based
- Licensed Enterprise (On-premise)
By Application
- Clinical Trials
- Clinical Research
- Others
By Clinical Trial Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By End-user
- Pharmaceutical & Biopharmaceutical Companies
- Contract Research Organizations (CROs)
- Academic Research Institutions
- Others
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
Clinical research is a sub-segment of medicine. It deals with researching and determining the efficiency of devices, medications, and diagnostic tools that are used in the healthcare industry.
The global e-clinical solutions market is expected to grow due to Need for efficient clinical trial management and regulatory compliance fuels growth. Rising R&D investments and cloud-based tools support adoption.
According to a study, the global e-clinical solutions market size was worth around USD 9.36 Billion in 2024 and is expected to reach USD 21.90 Billion by 2034.
The global e-clinical solutions market is expected to grow at a CAGR of 8.87% during the forecast period.
North America is expected to dominate the e-clinical solutions market over the forecast period.
Leading players in the global e-clinical solutions market include Oracle Corporation, Medidata Solutions Inc., ArisGlobal LLC, MedNet Solutions, DSG Inc., Parexel International Corporation, IBM Corporation, Bioclinica, CRF Health, Veeva Systems Inc., Datatrak International Inc., Bio-Optronics Inc., Forte Research Systems Inc., ERT Clinical, eClinical Solutions LLC, OmniComm Systems Inc., OpenClinica LLC, PRA Health Sciences Inc., Medable Inc., Merge Healthcare and many more., among others.
The report explores crucial aspects of the e-clinical solutions market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed