Biological Safety Testing Market Trend, Share, Growth, Size and Forecast 2032

Biological Safety Testing Market By Product (Instruments, Services and Kits, Reagent), By Type Of Test (Agents Detection Test, Cell Line Authentication and Characterization Tests, Bioburden Testing, Endotoxin Tests, Sterility Testing, Residual Host Contaminant Detection Tests, Other), By Application (Blood and Blood Products, Stem Cell Products, Cellular and Gene Therapy Products, Tissues and Tissue Products and Vaccines, Therapeutics), and By Region: Global Industry Perspective, Comprehensive Analysis and Forecast, 2024-2032
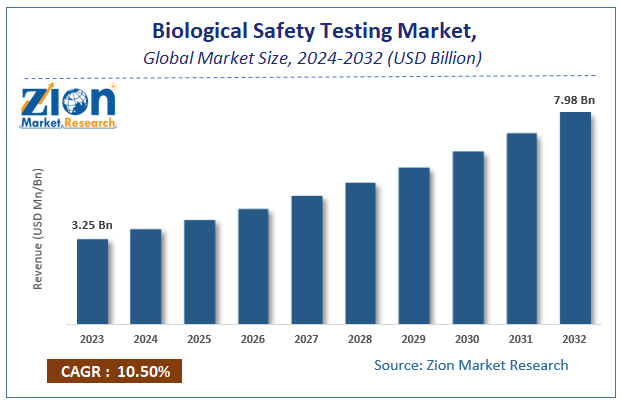
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 3.25 Billion | USD 7.98 Billion | 10.5% | 2023 |
Biological Safety Testing Market Size: Industry Perspective
The global Biological Safety Testing market size accrued earnings worth approximately USD 3.25 Billion in 2023 and is predicted to gain revenue of about USD 7.98 Billion by 2032, is set to record a CAGR of nearly 10.5% over the period from 2024 to 2032.
Global Biological Safety Testing Market: Overview
Biologics are products made from living organisms and have a unique set of properties. The safety of biologics is of utmost importance and specialized evaluation of the same is necessary. Biological safety testing is important for launching new products. Biological safety testing is done in order to ensure the safety quality and non-contamination of vaccines and biopharmaceuticals and to comply with the regulations for the same. Some of the major biological tests are sterility testing, residual host contaminant detection tests, bioburden testing, and endotoxin tests. Biological safety testing is used in the cellular, vaccine, and gene therapy products, tissue and tissue products, blood and blood products, and others. Better living standards, increased funding for life science research, and a rising number of pharmaceutical and biological firms are major factors that have been driving the biological safety testing market globally.
Global Biological Safety Testing Market: Growth Factors
The rising number of pharmaceutical and biological firms, the increase in government and private funds, and the growing number of biologics products are some of the major factors driving the biological safety testing market globally. Growth in the biological safety testing market will also be visible owing to a strong pharmaceutical product pipeline and increasing support from the government for biological and pharmaceutical industries. Regulatory bodies are working towards enforcing important standards for biological safety to ensure patient safety. Hence, the biological safety testing market has growing importance and has a potential for growth in the coming future. However, stringent regulations and the complicated procedure to obtain FDA approval may act as a restraining factor for the biological safety testing market.
Global Biological Safety Testing Market Report Scope:
| Report Attributes | Report Details |
|---|---|
| Report Name | Biological Safety Testing Market Research Report |
| Market Size in 2023 | USD 3.25 Billion |
| Market Forecast in 2032 | USD 7.98 Billion |
| Compound Annual Growth Rate | CAGR of 10.5% |
| Number of Pages | 210 |
| Forecast Units | Value (USD Billion), and Volume (Units) |
| Key Companies Covered | Charles River Laboratories International Inc., Sigma-Aldrich Corporation, Lonza Group, SGS SA, Avance Biosciences Inc., WuXiPharmaTech Inc., BSL Bioservice, Merck KGaA, Cytovance Biologics Inc., and Toxikon Corporation. |
| Segments Covered | By Test, By Product, By Application And By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East and Africa (MEA) |
| Countries Covered | North America: U.S and Canada Europe: Germany, Italy, Russia, U.K, Spain, France, Rest of Europe APAC: China, Australia, Japan, India, South Korea, South East Asia, Rest of Asia Pacific Latin America: Brazil, Argentina, Chile The Middle East And Africa: South Africa, GCC, Rest of MEA |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Biological Safety Testing Market: Segmentation
The global biological safety testing market is segmented into its product, type of test, application, and geography. Based on the product, the biological safety testing market is divided into instruments, services and kits, and reagents. Based on the type of test, the segregation is seen as an adventitious agents detection test, cell line authentication, and characterization test, bioburden testing, endotoxin tests, sterility testing, residual host contaminant detection tests, and other tests such as efficacy tests, stability tests, and toxicity test. On the basis of application, market segmentation is seen in blood and blood products, stem cell products, cellular and gene therapy products, tissues and tissue products, and vaccines and therapeutics. Regional diversification is seen in Asia, North America, Europe, and the Rest of the World.
Global Biological Safety Testing Market: Regional Analysis
Geographically, the global biological safety testing market is segmented into North America, Europe, Asia Pacific, and the Rest of the World. North America is the largest contender in the biological safety testing market. Countries such as Canada and the U.S. have major markets in the North American region. Rising demand for the biological safety testing market is also seen in the Asia Pacific region, especially in countries such as China, Japan, and India. China and India are the fastest-growing markets in the Asia-Pacific region. Demand for biological safety testing is seen to be steadily rising in Europe as well.
Global Biological Safety Testing Market: Competitive Players
Some of the major companies in the biological safety testing market are
- Charles River Laboratories International Inc.
- Sigma-Aldrich Corporation
- Lonza Group
- SGS SA
- Avance Biosciences Inc.
- WuXiPharmaTech Inc.
- BSL Bioservice
- Merck KGaA
- Cytovance Biologics Inc.
- Toxikon Corporation.
By Product
- Instruments
- Services and Kits
- Reagent
By Type Of Test
- Agents Detection Test
- Cell Line Authentication and Characterization Tests
- Bioburden Testing
- Endotoxin Tests
- Sterility Testing
- Residual Host Contaminant Detection Tests
- Other
By Application
- Blood and Blood Products
- Stem Cell Products
- Cellular and Gene Therapy Products
- Tissues and Tissue Products and Vaccines
- Therapeutics
Biological Safety Testing Market: Regional Segment Analysis
-
North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Rising number of pharmaceutical and biological firms, increase in government and private funds and growing number of biologics products are some of the major factors driving the biological safety testing market globally.
Global Biological Safety Testing market size earned around $3.25 Bn in 2023 and is expected to reach $7.98 Bn by 2032, with a projected CAGR of 10.5%.
North America is the largest contender in biological safety testing market. Countries such as Canada and the U.S. have the major markets in the North American region.
Some main participants of the biological safety testing market Charles River Laboratories International Inc., Sigma-Aldrich Corporation, Lonza Group, SGS SA, Avance Biosciences Inc., WuXiPharmaTech Inc., BSL Bioservice, Merck KGaA, Cytovance Biologics Inc., and Toxikon Corporation among others.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed