Asia-Pacific Diagnostic Tests Market Size, Share, Trends, Growth 2034

Asia-Pacific Diagnostic Tests Market By Test Type (Blood Tests, Urine Tests, Imaging, Genetic Tests, Biopsy, and Others), By Application (Infectious Diseases, Cancer, Cardiovascular, Diabetes, Neurology, and Others), By End-user (Hospitals, Clinics, Diagnostic Laboratories, Homecare, and Research Institutes), By Technology (PCR, ELISA, Rapid Test, Next-Generation Sequencing (NGS), and Imaging), and By Country: Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 197.41 Billion | USD 554.01 Billion | 10.87% | 2024 |
Asia-Pacific Diagnostic Tests Market: Industry Perspective
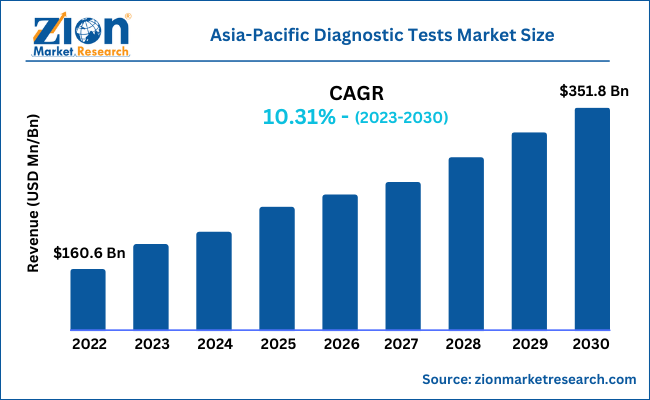
The Asia-Pacific diagnostic tests market size was worth around USD 197.41 billion in 2024 and is predicted to grow to around USD 554.01 billion by 2034 with a compound annual growth rate (CAGR) of roughly 10.87% between 2025 and 2034. The report analyzes the Asia-Pacific diagnostic tests market's drivers, restraints/challenges, and the effect they have on the demand during the projection period. Additionally, the report examines emerging opportunities in the Asia-Pacific diagnostic tests market.
Asia-Pacific Diagnostic Tests Market: Overview
The Asia Pacific diagnostic tests industry refers to the market for medical diagnostic tests in the Asia-Pacific region. It includes a wide range of diagnostic tests, such as clinical laboratory tests, imaging tests, molecular diagnostic tests, and others. The region in a broader sense covers the market in nations such as China, Japan, India, Australia, South Korea, and others. Most of these countries are densely populated and are home to some of the largest consumer groups in terms of population. This is one of the primary reasons why the region is witnessing a surge in quality medical care.
The Asia-Pacific region is highly competitive, especially in the healthcare sector and associated industries such as medical devices and equipment, due to the presence of numerous regional players along with the growing entry of international players. During the forecast period, the fraternity is expected to witness a steady growth rate, but it may face challenges and roadblocks due to several factors.
Key Insights:
- As per the analysis shared by our research analyst, the Asia-Pacific diagnostic tests market is estimated to grow annually at a CAGR of around 10.87% over the forecast period (2025-2034).
- Regarding revenue, the Asia-Pacific diagnostic tests market size was valued at around USD 197.41 billion in 2024 and is projected to reach USD 554.01 billion by 2034.
- The Asia-Pacific diagnostic tests market is projected to grow at a significant rate due to rising healthcare awareness, increasing chronic disease burden, expanding middle class, and government initiatives to improve diagnostic infrastructure across the region.
- Based on Test Type, the Blood Tests segment is expected to lead the market.
- On the basis of Application, the Infectious Diseases segment is growing at a high rate and will continue to dominate the market.
- Based on the End-user, the Hospitals segment is projected to swipe the largest market share.
- By Technology, the next-generation sequencing segment is expected to dominate the market.
- Based on region, Japan is predicted to dominate the market during the forecast period.
Asia-Pacific Diagnostic Tests Market: Growth Drivers
Growing prevalence of medical conditions is to propel market growth
The Asia-Pacific diagnostic tests market is projected to witness high growth owing to the increasing prevalence of several types of medical conditions. India and China are home to more than 50% of the global population. This means that the patient pool in these nations is higher than in most of the other countries. COVID-19 infections were a prime example of how easily the virus spread within a few months. For instance, on 7th May 2021, India reported about 414,188 new positive cases of Covid-19 infection, the highest in the country compared to 2020 and 2021 results.
However, it is crucial to note that the healthcare agencies in the region were swift in managing the condition by rolling out vaccines and awareness programs and leveraging digital technology to curb infection spread in the coming years. Similarly, other conditions are rapidly spreading in the Asia-Pacific region, which could lead to higher regional growth during the forecast period.
Asia-Pacific Diagnostic Tests Market: Restraints
Limited healthcare infrastructure to restrict market growth
The Asia Pacific diagnostic test industry may witness growth restrictions owing to the limited healthcare infrastructure in some Asian nations. Although several countries are investing heavily in improving healthcare and associated sectors, a major part of the regional economy has to deal with a serious lack of access to basic medical care. For instance, a 2020 report by the World Health Organization (WHO) suggested that nearly 65% of the population in Bangladesh lacks access to primary healthcare services.
Asia-Pacific Diagnostic Tests Market: Opportunities
Growing efforts toward self-dependence to provide growth opportunities
The Asia-Pacific diagnostic test market is expected to benefit from the shifting power dynamics between Western and Eastern nations across the globe. For instance, several Asian countries are working toward reducing their dependence on other nations, such as the US and European countries, for the import of advanced diagnostic tools. They are aiming at producing world-class devices domestically by assisting local manufacturers and suppliers. This could assist in higher growth in Asia-Pacific as countries become more self-reliant and reduce their cost of importing goods.
Asia-Pacific Diagnostic Tests Market: Challenges
Large market fragmentation to challenge market growth
The Asia-Pacific diagnostic tests market is highly fragmented due to the presence of several domestic or local stakeholders involved in the process. This acts as a challenge for the industry to manage its revenue as there is a need to streamline the process. The main concerns lie in market consolidation and competition, as fragmentation may lead to similar products circulating in the economy, leaving no scope for brand differentiation.
Asia-Pacific Diagnostic Tests Market: Segmentation Analysis
The Asia-Pacific diagnostic tests market is segmented based on test type, application, end-user, technology, and region.
Based on Test Type, the Asia-Pacific diagnostic tests market is divided into blood tests, urine tests, imaging, genetic tests, biopsy, and others.
On the basis of Application, the Asia-Pacific diagnostic tests market is bifurcated into infectious diseases, cancer, cardiovascular, diabetes, neurology, and others.
By End-user, the Asia-Pacific diagnostic tests market is split into hospitals, clinics, diagnostic laboratories, homecare, and research institutes. The hospitals segment is expected to witness the highest growth during the forecast period. These facilities provide excellent healthcare services and access to several diagnostic tools under one roof. They are also, generally, the first point of contact for the patients. Specialty clinics often tend to the needs of patients with specific conditions since they specialize in the treatment of these issues and the segment may generate high growth in the coming years. As per the WHO, India spent nearly 3.6% of its gross domestic product (GDP) on healthcare in 2020.
In terms of Technology, the Asia-Pacific diagnostic tests market is categorized into PCR, ELISA, rapid test, next-generation sequencing (NGS), and imaging. The next-generation sequencing (NGS) segment is expected to lead with the highest CAGR during the forecast period. The technology allows for sequencing large amounts of genetic material, which is used for the identification of genetic mutations that are related to specific conditions or diseases. The next-generation sequencing (NGS) process is highly cost-effective and has the potential to be used for promoting personalized medical treatment. Other segments, such as spectroscopy-based, immunoassay-based substrate technology, and microfluidics, also have growth potential. As per official records, China spent nearly USD 19.2 billion on its medical device industry in 2020.
Asia-Pacific Diagnostic Tests Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Asia-Pacific Diagnostic Tests Market |
| Market Size in 2024 | USD 197.41 Billion |
| Market Forecast in 2034 | USD 554.01 Billion |
| Growth Rate | CAGR of 10.87% |
| Number of Pages | 190 |
| Key Companies Covered | Mindray Medical International Limited, Roche Diagnostics, Bio-Rad Laboratories, Abbott Laboratories, Becton, Dickinson and Company, Siemens Healthineers, F. Hoffmann-La Roche Ltd., Danaher Corporation, QIAGEN N.V., Thermo Fisher Scientific, Beckman Coulter Inc., Sysmex Corporation, Bio-Rad Laboratories, Hologic Inc., Ortho Clinical Diagnostics, Cepheid Inc., Luminex Corporation, Meridian Bioscience Inc., Eiken Chemical Co. Ltd., Nihon Kohden Corporation, Alere Inc., BioMérieux SA, DiaSorin S.p.A., Fujirebio Inc., and Grifols S.A., and others. |
| Segments Covered | By Test Type, By Application, By End-user, By Technology, and By Region |
| Regions Covered | Asia Pacific (APAC) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Asia-Pacific Diagnostic Tests Market: Regional Analysis
Japan is to witness a high growth rate during the coming period
The regional Asia-Pacific diagnostic tests market is expected to witness tremendous growth in Japan owing to the robust medical and healthcare infrastructure of the island nation. Several factors have led to the small economy providing excellent medical assistance to its population. One such aspect is the presence of universal healthcare coverage, which means that all of the country’s residents have equal access to affordable healthcare irrespective of age, income, and health status.
This ensures that patients do not have to worry about the monetary aspects of their health. Japan has always focused on preventive care by conducting regular screenings and check-ups for its residents. This allows them to detect conditions in their early stages, letting them provide accurate care. In addition to this, the growing investments toward improving healthcare services could lead to more growth.
Recent Developments
- In December 2022, PredOmix, an Indian start-up, unveiled OncoVeryx-F. The product is a one-of-a-kind blood test for detecting cancer. It can detect the deadly disease in its early stages with an accuracy rate of almost 98%. The test is powered using artificial intelligence (AI) and advanced metabolomics technology
- In June 2022, Apollo Cancer Center, the research wing of the Indian multinational healthcare group, launched a new blood test for the early detection of breast cancer. Experts have suggested that additional complementary tests would be needed to confirm the accuracy of the test. The blood test is called EasyCheck-Breast
- In August 2021, Berry Oncology, a Berry Genomics subsidiary, accelerated its research on ways to detect liver cancer in its early stages. The screening technique being researched is based on NGS technology
Asia-Pacific Diagnostic Tests Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the Asia-Pacific diagnostic tests market on a regional basis.
The Asia-Pacific diagnostic tests market is dominated by players like:
- Mindray Medical International Limited
- Roche Diagnostics
- Bio-Rad Laboratories
- Abbott Laboratories
- Becton
- Dickinson and Company
- Siemens Healthineers
- F. Hoffmann-La Roche Ltd.
- Danaher Corporation
- QIAGEN N.V.
- Thermo Fisher Scientific
- Beckman Coulter Inc.
- Sysmex Corporation
- Bio-Rad Laboratories
- Hologic Inc.
- Ortho Clinical Diagnostics
- Cepheid Inc.
- Luminex Corporation
- Meridian Bioscience Inc.
- Eiken Chemical Co., Ltd.
- Nihon Kohden Corporation
- Alere Inc.
- BioMérieux SA
- DiaSorin S.p.A.
- Fujirebio Inc.
- and Grifols S.A.
The Asia-Pacific diagnostic tests market is segmented as follows;
By Test Type
- Blood Tests
- Urine Tests
- Imaging
- Genetic Tests
- Biopsy
- Others
By Application
- Infectious Diseases
- Cancer
- Cardiovascular
- Diabetes
- Neurology
- Others
By End-user
- Hospitals
- Clinics
- Diagnostic Laboratories
- Homecare
- Research Institutes
By Technology
- PCR
- ELISA
- Rapid Test
- Next-Generation Sequencing (NGS)
- Imaging
By Region
- Asia Pacific
- China
- Japan
- India
- New Zealand
- Australia
- South Korea
- Southeast Asia
- Rest of Asia Pacific
Table Of Content
Methodology
FrequentlyAsked Questions
The industry refers to the market for medical diagnostic tests in the Asia-Pacific region. It includes a wide range of diagnostic tests, such as clinical laboratory tests, imaging tests, molecular diagnostic tests, and others.
The Asia-Pacific diagnostic tests market is projected to witness high growth owing to the increasing prevalence of several types of medical conditions. India and China are home to more than 50% of the global population.
According to a study, the Asia-Pacific diagnostic tests market size was worth around USD 197.41 billion in 2024 and is expected to reach USD 554.01 billion by 2034.
The Asia-Pacific diagnostic tests market is expected to grow at a CAGR of 10.87% during the forecast period.
The regional Asia-Pacific diagnostic tests market is expected to witness tremendous growth in Japan owing to the robust medical and healthcare infrastructure of the island nation.
Leading players in the Asia-Pacific diagnostic tests market include Mindray Medical International Limited, Roche Diagnostics, Bio-Rad Laboratories, Abbott Laboratories, Becton, Dickinson and Company, Siemens Healthineers, F. Hoffmann-La Roche Ltd., Danaher Corporation, QIAGEN N.V., Thermo Fisher Scientific, Beckman Coulter Inc., Sysmex Corporation, Bio-Rad Laboratories, Hologic Inc., Ortho Clinical Diagnostics, Cepheid Inc., Luminex Corporation, Meridian Bioscience Inc., Eiken Chemical Co. Ltd., Nihon Kohden Corporation, Alere Inc., BioMérieux SA, DiaSorin S.p.A., Fujirebio Inc., and Grifols S.A., among others.
The report explores crucial aspects of the Asia-Pacific diagnostic tests market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed