Mycoplasma Detection System Market Size, Share, Growth & Forecast 2034

Mycoplasma Detection System Market By Product (Instruments, Kits & Reagents, and Services), By Technology (PCR, ELISA, Direct Assay, Indirect Assay, Microbial Culture Techniques, and Enzymatic Methods), By Application (Cell Line Testing, Virus Testing, End of Production Cells Testing, and Others), By End-Use (Academic Research Institutes, Cell Banks, Contract Research Organizations, Pharmaceutical & Biotechnology Companies, and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
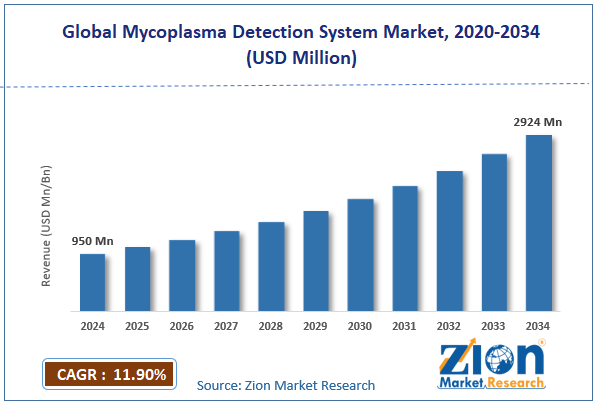
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 950 Million | USD 2924 Million | 11.9% | 2024 |
Mycoplasma Detection System Industry Perspective:
The global mycoplasma detection system market size was worth around USD 950 million in 2024 and is predicted to grow to around USD 2924 million by 2034, with a compound annual growth rate (CAGR) of roughly 11.9% between 2025 and 2034.
Key Insights
- As per the analysis shared by our research analyst, the global mycoplasma detection system market is estimated to grow annually at a CAGR of around 11.9% over the forecast period (2025-2034).
- In terms of revenue, the global mycoplasma detection system market size was valued at around USD 950 million in 2024 and is projected to reach USD 2924 million by 2034.
- The growing pharmaceutical and biotechnology sector is expected to drive the mycoplasma detection system market over the forecast period.
- Based on the product, the kits & reagents segment is expected to capture the largest market share over the projected period.
- Based on the technology, the PCR segment is expected to capture the largest market share over the projected period.
- Based on the application, the virus testing segment is expected to capture the largest market share over the projected period.
- Based on the end-use, the pharmaceutical & biotechnology companies segment is expected to capture the largest market share over the projected period.
- Based on region, North America is expected to dominate the market during the forecast period.
Mycoplasma Detection System Market: Overview
A mycoplasma detection system is a specialized analytical platform that can detect mycoplasma contamination in cell cultures, biopharmaceutical products, vaccines, and research laboratories. These systems use PCR, DNA staining, ELISA, culture-based assays, or enzymatic bioluminescence to find mycoplasma. Mycoplasma are tiny bacteria that lack cell walls and can alter the way cells grow and the outcome of experiments. Market growth is being driven by several factors, including the increasing use of cell culture in biopharmaceuticals and research, stringent regulations and quality standards, a shift from traditional culture methods to rapid detection, and others. But a lack of skilled workers makes it hard for the business to grow.
Mycoplasma Detection System Market Dynamics
Growth Drivers
How does increasing use of cell culture in biopharma & research drive the mycoplasma detection system market growth?
The mycoplasma detection system market is driven by the increasing use of cell culture in biopharmaceuticals and research, as cell cultures are particularly susceptible to mycoplasma contamination, which can significantly impact cell viability, metabolism, and experimental outcomes. The need for large-scale, accurate, and contamination-free cell culture technologies is growing in tandem with the global demand for biopharmaceuticals, including monoclonal antibodies, vaccines, stem cell therapies, and gene therapies.
The biopharmaceutical industry is experiencing rapid growth and is projected to generate tens of billions of dollars by the mid-2030s. It relies heavily on mammalian and other cell cultures to make biologics and vaccines. Any contamination with mycoplasma could ruin these expensive and complex manufacturing processes; therefore, they need to be tested frequently and strictly. As a result, the sector continues to grow.
Restraints
Why does the high cost of advanced detection systems impede the mycoplasma detection system market growth?
The high cost of advanced mycoplasma detection systems makes it challenging for the mycoplasma detection system market to grow, as it hinders many potential customers, including small and medium-sized biotechnology enterprises, contract research organizations, academic labs, and new market facilities, from affording them. PCR-based approaches and other advanced Mycoplasma detection kits and reagents are pretty expensive.
For instance, individual detection kits can cost anywhere from $200 to $3,000, and complete PCR systems require the acquisition of expensive equipment that works with them. This makes regular testing costly, a problem for businesses with limited budgets. Additionally, automated detection systems can cost between $100,000 and $500,000 or more, and annual maintenance and service contracts can incur an additional $50,000 or more. Smaller labs and research groups struggle to justify such high capital costs; therefore, they often put off or postpone upgrading to automated, efficient systems.
Opportunities
Does the implementation of stringent regulatory standards offer a lucrative opportunity for the mycoplasma detection system industry’s growth?
Regulatory bodies have established strict rules and criteria for mycoplasma testing to ensure that products meet these standards and are safe for use. This is what is driving the industry. Businesses that require strict quality control are increasingly utilizing mycoplasma testing methods and technologies. The market for Mycoplasma testing has grown as testing methods continue to improve. For instance, PCR-based tests with higher sensitivity and specificity have made it easier to find mycoplasma contamination. Thus, the implementation of stringent regulatory standards offers a potential opportunity to the mycoplasma detection system sector.
Challenges
Shortage of skilled professionals poses a major challenge to market expansion
The lack of qualified employees is a significant barrier to the growth of the mycoplasma detection system market, as these technologies require specialized skills to operate, validate, and interpret results. To utilize advanced molecular detection methods, such as PCR and digital PCR, one requires trained molecular quality assurance staff, which is currently in short supply. Mycoplasma detection technologies, particularly PCR-based and automated systems, require complex protocols and a high level of technical expertise to ensure accurate testing and adherence to regulations. Not many labs can employ these technologies since there aren't enough skilled people.
Mycoplasma Detection System Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Mycoplasma Detection System Market |
| Market Size in 2024 | USD 950 Million |
| Market Forecast in 2034 | USD 2924 Million |
| Growth Rate | CAGR of 11.9% |
| Number of Pages | 214 |
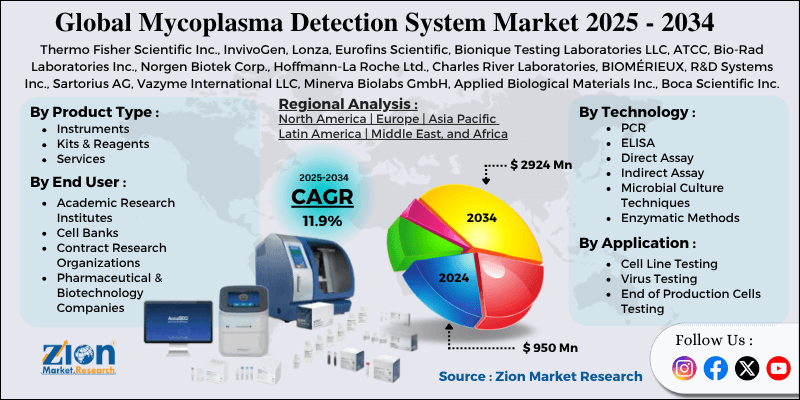
| Key Companies Covered | Thermo Fisher Scientific Inc., InvivoGen, Lonza, Eurofins Scientific, Bionique Testing Laboratories LLC, ATCC, Bio-Rad Laboratories Inc., Norgen Biotek Corp., Hoffmann-La Roche Ltd., Charles River Laboratories, BIOMÉRIEUX, R&D Systems Inc., Sartorius AG, Vazyme International LLC, Minerva Biolabs GmbH, Applied Biological Materials Inc., Boca Scientific Inc., GeneCopoeia Inc., and others. |
| Segments Covered | By Product, By Technology, By Application, By End-Use, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Mycoplasma Detection System Market: Segmentation
The global mycoplasma detection system industry is segmented based on product, technology, application, end-use, and region.
Based on the product, the global mycoplasma detection system market is bifurcated into instruments, kits & reagents, and services. The kits & reagents segment is expected to capture the largest market share over the projected period. Kits and reagents, unlike instruments, are consumable items that must be purchased each time a mycoplasma test is performed. This provides a consistent and rising revenue stream as biopharma and research labs ramp up testing frequency, driving segment expansion.
Based on the technology, the global mycoplasma detection system industry is bifurcated into PCR, ELISA, direct assay, indirect assay, microbial culture techniques, and enzymatic methods. The PCR segment holds the major market share. PCR-based tests can deliver results in hours rather than the days or weeks required for culture-based methods, facilitating quicker contamination control decisions and reducing production downtime.
Based on the application, the global mycoplasma detection system market is bifurcated into cell line testing, virus testing, end of production cells testing, and others. The virus testing segment is expected to hold the largest market share over the projected period. Virus testing forms a significant application segment within this market, driven by contamination concerns in virus-based biologics and vaccines.
Based on the end-use, the global mycoplasma detection system industry is bifurcated into academic research institutes, cell banks, contract research organizations, pharmaceutical & biotechnology companies, and others. The pharmaceutical & biotechnology companies’ segment is expected to dominate the market during the projected period. The increasing pharmaceutical and biotechnology company and the growing launches of several vaccines drives the industry growth.
Mycoplasma Detection System Market: Regional Analysis
Why does North America dominate the mycoplasma detection system market over the projected period?
The North America region is expected to dominate the mycoplasma detection system market. There are numerous important biopharma and biotech centers in North America, particularly in the United States. Boston, San Francisco, and the Research Triangle are among the most notable ones. These places have a robust pharmaceutical infrastructure and cutting-edge mycoplasma detection technologies to ensure that quality control and regulatory compliance are met. The FDA also demands thorough checks for contamination in the research and production of biopharmaceuticals.
Due to this, modern molecular techniques such as PCR are now widely used to detect Mycoplasma. In addition, top global firms such as Thermo Fisher Scientific, Lonza, Sartorius, and Charles River have established strong facilities in North America, offering cutting-edge detection solutions that help drive market growth.
Mycoplasma Detection System Market: Competitive Analysis
The global mycoplasma detection system market is dominated by players like:
- Thermo Fisher Scientific Inc.
- InvivoGen
- Lonza
- Eurofins Scientific
- Bionique Testing Laboratories LLC
- ATCC
- Bio-Rad Laboratories Inc.
- Norgen Biotek Corp.
- Hoffmann-La Roche Ltd.
- Charles River Laboratories
- BIOMÉRIEUX
- R&D Systems Inc.
- Sartorius AG
- Vazyme International LLC
- Minerva Biolabs GmbH
- Applied Biological Materials Inc.
- Boca Scientific Inc.
- GeneCopoeia Inc.
The global mycoplasma detection system market is segmented as follows:
By Product
- Instruments
- Kits & Reagents
- Services
By Technology
- PCR
- ELISA
- Direct Assay
- Indirect Assay
- Microbial Culture Techniques
- Enzymatic Methods
By Application
- Cell Line Testing
- Virus Testing
- End of Production Cells Testing
- Others
By End-Use
- Academic Research Institutes
- Cell Banks
- Contract Research Organizations
- Pharmaceutical & Biotechnology Companies
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
A mycoplasma detection system is a specialized analytical platform that can detect mycoplasma contamination in cell cultures, biopharmaceutical products, vaccines, and research laboratories.
The mycoplasma detection system market growth is being driven by several factors, including the increasing use of cell culture in biopharmaceuticals and research, stringent regulations and quality standards, a shift from traditional culture methods to rapid detection, and others.
The lack of a skilled workforce poses a major challenge to the industry's expansion.
Based on the application, the virus testing segment is expected to dominate the mycoplasma detection system market growth during the projected period.
The increasing trends of personalized medicine pose a major impact on the mycoplasma detection system industry's growth over the projected period.
According to the report, the global mycoplasma detection system market size was worth around USD 950 million in 2024 and is predicted to grow to around USD 2924 million by 2034.
The global mycoplasma detection system market is expected to grow at a CAGR of 11.9% during the forecast period.
The global mycoplasma detection system industry growth is expected to be driven by the North America region. It is currently the world’s highest revenue-generating market due to the developed pharmaceutical sector and the presence of major players.
The global mycoplasma detection system market is dominated by players like Thermo Fisher Scientific Inc., InvivoGen, Lonza, Eurofins Scientific, Bionique Testing Laboratories LLC, ATCC, Bio-Rad Laboratories Inc., Norgen Biotek Corp., Hoffmann-La Roche Ltd., Charles River Laboratories, BIOMÉRIEUX, R&D Systems, Inc., Sartorius AG, Vazyme International LLC, Minerva Biolabs GmbH, Applied Biological Materials Inc., Boca Scientific Inc., and GeneCopoeia, Inc., among others.
The mycoplasma detection system market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTLE analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five forces analysis, and value chain analysis.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed