Duchenne Muscular Dystrophy Drugs Market Size, Share, Trends, Growth and Forecast 2034

Duchenne Muscular Dystrophy Drugs Market By Product Type (Corticosteroids, Pain Management Drugs), By therapeutic Approach (Mutation Suppression, Exon Skipping, Steroid Therapy), By End User (Clinics, Hospitals, Home Care Settings), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 4.05 Billion | USD 14.04 Billion | 16.80% | 2024 |
Duchenne Muscular Dystrophy Drugs Industry Perspective:
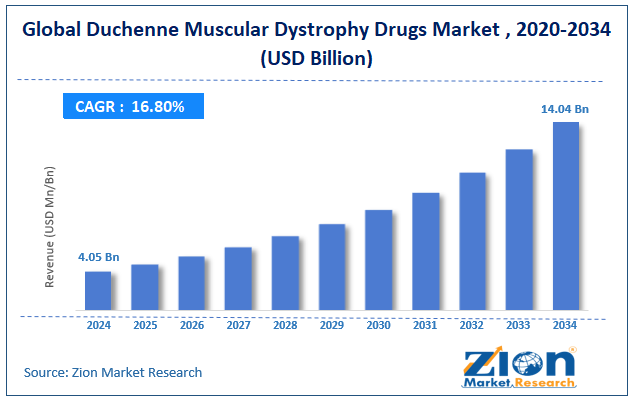
The global Duchenne muscular dystrophy drugs market size was worth around USD 4.05 billion in 2024 and is predicted to grow to around USD 14.04 billion by 2034, with a compound annual growth rate (CAGR) of roughly 16.80% between 2025 and 2034.
Duchenne Muscular Dystrophy Drugs Market: Overview
Duchenne muscular dystrophy drugs are dedicated medicines to slow disease progression, enhance quality of life, and manage symptoms for individuals suffering from this severe genetic condition. DMD is caused by changes in the dystrophin gene, resulting in progressive muscle weakness and degeneration, especially in boys.
The worldwide Duchenne muscular dystrophy drugs market is anticipated to grow significantly over the coming years, driven by the increasing number of DMD cases globally, advancements in molecular therapies, and the rapid approval of targeted therapies.
The growing instances of Duchenne muscular dystrophy, mainly among newborn males, are fueling the demand for drugs. DMD affects 1 in every 3,500 – 5,000 live male births. This growing patient population increases the demand for efficient treatment and long-term management of the disease.
Innovations in exon-skipping solutions and gene editing are transforming treatment possibilities. Drugs like golodirsen and eteplirsen have gained speedy approvals because of results in restoring dystrophin. These modernizations offer a new ray of hope, motivating investments in research and development.
Moreover, the latest FDA acceptance of disease-modifying therapies has improved industry activity. For example, Sarepta Therapeutics has gained regulatory approval for multiple exon-skipping medications. These targeted treatments grow therapeutic options, fueling commercial growth.
Nevertheless, some restraining factors impacting market progress include the significantly priced DMD therapies and a restricted patient pool. Several DMD medications, mainly non-skipping therapies, cost over USD 3,00,000 yearly for every patient. The high cost restricts its availability, mainly in developing and low-income nations.
Even with insurance policies, co-pays may be unreasonable for most families. Also, DMD is a rare illness, which limits the target patient pool. This restricts the potential return on investment (ROI) for novel drug development. Therefore, most companies are hesitant to invest in niche treatment, notwithstanding medical demands.
However, the global Duchenne muscular dystrophy drugs industry is expected to experience substantial growth in the coming years, driven by advancements in exon skipping technology, precision therapy, and personalized medicine.
Fresh discoveries in exon skipping may address changes in the dystrophin gene. Currently, therapies only encompass a few exons, such as 54, 53, and 51. Broader exon coverage may expand the pool of patients who can be treated.
Additionally, advancements in biomarker research and genome sequencing are enabling the development of patient-specific treatment options. Personalized approaches may minimize ill effects and improve drug efficacy. This offers opportunities for high-impact and premium-priced therapeutics.
Key Insights:
- As per the analysis shared by our research analyst, the global Duchenne muscular dystrophy drugs market is estimated to grow annually at a CAGR of around 16.80% over the forecast period (2025-2034)
- In terms of revenue, the global Duchenne muscular dystrophy drugs market size was valued at around USD 4.05 billion in 2024 and is projected to reach USD 14.04 billion by 2034.
- The Duchenne muscular dystrophy drugs market is projected to grow significantly owing to the rising disease pressure of DMD, growing awareness and diagnosis of DMD, and improvements in genetic research.
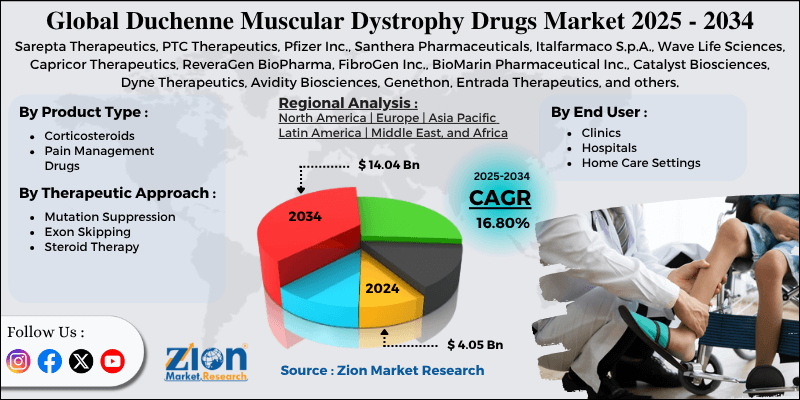
- Based on product type, the corticosteroids segment is expected to lead the market, while the pain management drugs segment is expected to grow considerably.
- Based on the therapeutic approach, the steroid therapy segment is the dominating segment, while the exon skipping segment is projected to witness sizeable revenue over the forecast period.
- Based on end user, the hospitals segment is expected to lead the market compared to the clinics segment.
- Based on region, North America is projected to dominate the global market during the estimated period, followed by Asia Pacific.
Duchenne Muscular Dystrophy Drugs Market: Growth Drivers
Growing adoption of biomarker development and personalized medicine drives market growth
Personalized medicine is emerging as the foundation for efficient management of DMD, driven by advancements in genetic profiling and biomarker identification. By modifying treatments to specific patient profiles and mutations, personalized therapies reduce ill effects and improve efficiency.
Recent studies have identified novel biomarkers, such as serum creatine kinase levels and microRNAs, that aid in monitoring treatment response and disease progression. This accurate approach is widely adopted in routine care and clinical trials, as evidenced by the increasing number of biomarker-based DMD analyses registered in 2025.
Improvements in novel treatment approaches and gene therapy fuel the market growth
Innovations in exon skipping, gene therapy, and genome editing solutions are among the strongest propellers in the global Duchenne muscular dystrophy drugs market.
For example, the acceptance of gene therapy candidates, such as Wave Life Sciences' investigational therapies and Sarepta Therapeutics’ Exondys 51, has generated new treatment possibilities targeting fundamental genetic mutations.
Recent news indicates that CRISPR-Cas9-enabled therapies are entering early-stage clinical trials, promising permanent solutions by correcting mutations at the DNA level.
Duchenne Muscular Dystrophy Drugs Market: Restraints
Low awareness and diagnosis in developing markets negatively impact market progress
While awareness of DMD is increasing in emerging economies, several developing regions continue to face challenges related to patient identification and diagnosis. The lack of specialized diagnostic infrastructure and restricted access to genetic testing hampers early detection.
Current data from the WHO suggests that in areas like Sub-Saharan Africa and Southeast Asia, over 60 percent of potential DMD numbers are misdiagnosed or undiagnosed because of inadequate healthcare technologies and services. This restricts the patient pool for therapies and hinders the growth of the Duchenne muscular dystrophy drugs industry in these locations.
Duchenne Muscular Dystrophy Drugs Market: Opportunities
Increasing regulatory incentives and orphan drug designations positively impact market growth
The orphan drug regulatory architecture offers key benefits, including market exclusivity, fee waivers, and tax credits, which incentivize the production of rare disease drugs. The FDA has granted orphan status to more than 20 new DMD drug candidates, indicating a rise in pipeline activity.
The growing regulatory incentives in developing markets like India and China are also attracting new players and entrants, thus impacting the growth of the Duchenne muscular dystrophy drugs market.
For instance, China's NMPA (National Medical Products Administration) has simplified the approval process for orphan drugs since 2023, creating a key opportunity to reach underserved and large populations.
Duchenne Muscular Dystrophy Drugs Market: Challenges
Difficulties in drug delivery to target tissues restrict the growth of market
Delivering therapeutic agents efficiently to muscle tissues in the body is a key technical challenge. DMD affects multiple muscle groups, like cardiac muscles, making systemic delivery challenging but essential.
Presently available delivery techniques demand intramuscular or intravenous administration with frequent dosage, which is pressurizing for progressive patients.
According to a patient care survey by Global Genes in 2024, over 55% of caregivers reported challenges with their current treatment routines, which adversely impacted outcomes and compliance.
Duchenne Muscular Dystrophy Drugs Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Duchenne Muscular Dystrophy Drugs Market |
| Market Size in 2024 | USD 4.05 Billion |
| Market Forecast in 2034 | USD 14.04 Billion |
| Growth Rate | CAGR of 16.80% |
| Number of Pages | 211 |
| Key Companies Covered | Sarepta Therapeutics, PTC Therapeutics, Pfizer Inc., Santhera Pharmaceuticals, Italfarmaco S.p.A., Wave Life Sciences, Capricor Therapeutics, ReveraGen BioPharma, FibroGen Inc., BioMarin Pharmaceutical Inc., Catalyst Biosciences, Dyne Therapeutics, Avidity Biosciences, Genethon, Entrada Therapeutics, and others. |
| Segments Covered | By Product Type, By Therapeutic Approach, By End User, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Duchenne Muscular Dystrophy Drugs Market: Segmentation
The global Duchenne muscular dystrophy drugs market is segmented based on product type, therapeutic approach, end user, and region.
Based on product type, the global industry is divided into corticosteroids and pain management drugs. The corticosteroids segment has registered a remarkable market share, as they are widely prescribed medicines for DMD. This is because of their ability to slow muscle degeneration, delay complications, and preserve ambulation. Their established efficiency, broad availability, and comparatively low price increase their demand for treating Duchenne muscular dystrophy (DMD). According to recent assessments, corticosteroids are the leading treatment due to their prolonged clinical use and guideline-based recommendations.
Based on the therapeutic approach, the global Duchenne muscular dystrophy drugs market is segmented into mutation suppression, exon skipping, and steroid therapy. The steroid therapy segment is still the broadly used therapeutic approach in managing DMD. Drugs like deflazacort are the best practice since they postpone ambulation loss, delay disease progression, and preserve muscle strength. Steroids are given to all diagnosed individuals, increasing their accessibility and affordability on a global scale, mainly in middle and low-income nations.
Based on end user, the global market is segmented into clinics, hospitals, and home care settings. The hospitals segment captured a significant share of the market due to their ability to manage multidisciplinary and complex care. Several genetic tests, initial diagnoses, advanced treatments, and steroid therapy initiation are performed in hospitals. Their availability of pediatric neurologists, access to emergency care, and integrated infrastructure make hospitals key centers for handling severe and moderate DMD cases.
Duchenne Muscular Dystrophy Drugs Market: Regional Analysis
North America to witness significant growth over the forecast period
North America is anticipated to hold strong leadership in the Duchenne muscular dystrophy drugs market due to its high diagnosis and awareness ratio, the presence of prominent DMD drug producers, and a robust clinical trial and R&D network. North America, mainly the United States, holds the leading rate of early DMD diagnosis because of newborn screening initiatives, awareness among healthcare providers, and access to genetic testing.
Moreover, the region is home to key DMD drug producers like PTC Therapeutics, Sarepta Therapeutics, and Pfizer, which lead worldwide commercial operations and clinical trials from the United States. For example, in 2023, Sarepta reported over USD 800 million in revenue, majorly from DMD therapies. Their production capabilities and headquarters are based in North America, strengthening their regional prominence.
Furthermore, the region registers a notable share of the global clinical trials for rare diseases, comprising DMD. As of 2024, over 60 percent of all active DMD clinical trials were conducted in the United States. This strong partnership between academia and biotech boosts drug discovery and pipeline growth in the region.
The Asia Pacific Duchenne muscular dystrophy drugs industry holds a considerable share after North America, driven by the rising patient pool, enhanced healthcare infrastructure, and favorable government policies. The Asia Pacific region has a growing base of DMD patients, primarily in populous nations such as China and India.
Surveys suggest that India alone registers over 30,000 boys suffering from DMD, with thousands of fresh cases reported every year. This mounting birth rate and large pediatric population support the regional demand for DMD treatments.
Moreover, healthcare infrastructure is speedily growing in emerging nations, with governments heavily investing in rare disease care. The National Rare Disease Registry System in China is enhancing patient access to drugs and improving tracking. Japan has incorporated DMD in its insurance coverage. These advancements are increasing the accessibility of the said drugs at specialized clinics and hospitals.
Governments in the region are expanding their policy architecture for rare diseases. This enhances drug approval and reimbursement schedules, aiding funding and patient support, thereby fueling industry growth in these regions.
Duchenne Muscular Dystrophy Drugs Market: Competitive Analysis
The leading players in the global Duchenne muscular dystrophy drugs market are:
- Sarepta Therapeutics
- PTC Therapeutics
- Pfizer Inc.
- Santhera Pharmaceuticals
- Italfarmaco S.p.A.
- Wave Life Sciences
- Capricor Therapeutics
- ReveraGen BioPharma
- FibroGen Inc.
- BioMarin Pharmaceutical Inc.
- Catalyst Biosciences
- Dyne Therapeutics
- Avidity Biosciences
- Genethon
- Entrada Therapeutics
Duchenne Muscular Dystrophy Drugs Market: Key Market Trends
- Growing focus on newborn screening and early diagnosis:
Advanced genetic testing and early detection via newborn screening programs are gaining popularity as a vital trend. Healthcare providers and countries are heavily investing in early diagnosis to allow timely treatment initiation, enhancing patient outcomes. This early detection trend fuels the demand for disease-modifying medications rather than merely supportive care.
- The inclination toward advanced genetic treatments and gene therapy:
There is a strong industry shift towards exon-skipping drugs and gene therapy. FDA approvals of therapies like eteplirsen and Elevidys underscore this inclination. These treatments address the root genetic cause of Duchenne muscular dystrophy, providing potentially durable and personalized benefits than symptomatic therapies.
The global Duchenne muscular dystrophy drugs market is segmented as follows:
By Product Type
- Corticosteroids
- Pain Management Drugs
By Therapeutic Approach
- Mutation Suppression
- Exon Skipping
- Steroid Therapy
By End User
- Clinics
- Hospitals
- Home Care Settings
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Duchenne muscular dystrophy drugs are dedicated medicines to slowing disease progression, enhancing quality of life, and managing symptoms for individuals suffering from this severe genetic condition. DMD is caused by changes in the dystrophin gene, resulting in progressive muscle weakness and degeneration, especially in boys.
The global Duchenne muscular dystrophy drugs market is projected to grow due to increasing research funding and activities, rising approvals of targeted therapies, and advancements in drug delivery.
According to study, the global Duchenne muscular dystrophy drugs market size was worth around USD 4.05 billion in 2024 and is predicted to grow to around USD 14.04 billion by 2034.
The CAGR value of the Duchenne muscular dystrophy drugs market is expected to be around 16.80% during 2025-2034.
North America is expected to lead the global Duchenne muscular dystrophy drugs market during the forecast period.
The key players profiled in the global Duchenne muscular dystrophy drugs market include Sarepta Therapeutics, PTC Therapeutics, Pfizer Inc., Santhera Pharmaceuticals, Italfarmaco S.p.A., Wave Life Sciences, Capricor Therapeutics, ReveraGen BioPharma, FibroGen, Inc., BioMarin Pharmaceutical Inc., Catalyst Biosciences, Dyne Therapeutics, Avidity Biosciences, Genethon, and Entrada Therapeutics.
The report examines key aspects of the Duchenne muscular dystrophy drugs market, including a detailed analysis of existing growth factors and restraints, as well as an exploration of future growth opportunities and challenges that influence the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed