Transcathetar Devices Market Size, Share, And Growth Report 2032

Transcathetar Devices Market by Product (Transcathetar Embolization and Occlusion Devices, Transcathetar replacement devices and Transcathetar replacement devices), by application (Cardiovascular, Oncology, Neurology, Urology and Others), By Region - Global And Regional Industry Overview, market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1198.34 Million | USD 2830.24 Million | 10.02% | 2023 |
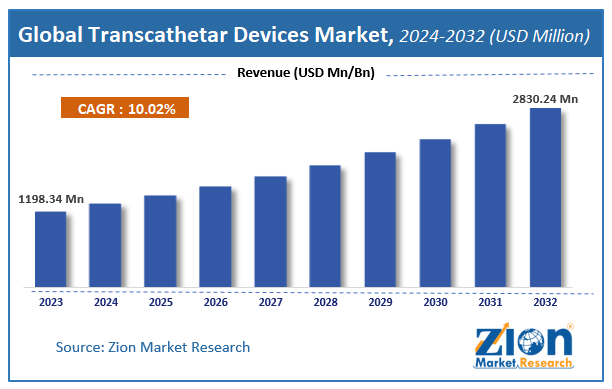
The global Transcathetar Devices market size was worth around USD 1198.34 million in 2023 and is predicted to grow to around USD 2830.24 million by 2032 with a compound annual growth rate (CAGR) of roughly 10.02% between 2024 and 2032.
The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD million). The report covers a forecast and an analysis of the Transcathetar Devices market on a global and regional level.
Transcathetar Devices Market: Overview
Transcathetar devices include treatment such as TAVR, TAVI, TMVR, and TMVI. Approaches to the Transcathetar technique include transfemoral, transapical and transaortic procedures. This report is an analytical business tool with the primary purpose of providing a comprehensive global market evaluation for heart valve disease transcatheter treatment.
Favorable insurance policies and compensation scenario are one of the key factors expected to drive the sector in the years to come. Some of the factors that contribute to increased coverage are the increasing number of approvals and the increasing number of sponsors for TAVR and TMVR procedures in this region. For example, the Centers for Medicare & Medicaid Services (CMS) confirmed it had approved TAVR coverage under the Medicare National Coverage Determination Strategy. Various efforts are underway to raise awareness of TAVR and TMVR procedures. The STS / ACC TVT Registry is a joint project of the Society of Thoracic Surgeons (STS) and American College of Cardiology to track real-world outcomes and patient safety related to repair and replacement surgery of transcatheter valves. CMS supports this list which offers information into patient outcomes and recommendations for procedures for replacement and repair.
The study includes drivers and restraints for the Transcathetar Devices market along with the impact they have on the demand over the forecast period. Additionally, the report includes the study of opportunities available in the Transcathetar Devices market on a global level. In order to give the users of this report a comprehensive view on the Transcathetar Devices market we have included competitive landscape and analysis of Porter’s Five Forces model for the market. The study encompasses a market attractiveness analysis, wherein product, application, and regional segments are benchmarked based on their market size, growth rate and general attractiveness.
Transcathetar Devices Market: Segmentation
The study provides a decisive view on the Transcathetar Devices market by segmenting the market based on product, application and regions. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032.
Based on product, the market is segmented into Transcathetar Embolization and Occlusion Devices, Transcathetar replacement devices and Transcathetar replacement devices. It is projected that the Transcathetar replacement Devices segment will dominate the largest share of the Transcathetar Devices market in 2023. The Transcatheter Aortic Valve Replacement (TAVR) devices led by Transcatheter Mitral Valve Replacement represent the highest revenue share of replacement devices due to increasing mergers & acquisitions, partnerships, and R&D funding to provide effective treatment and minimize complications. Most of these devices are expected to improve the treatment of patients suffering from regurgitation of the mitral valve and global symptomatic aortic valve stenosis. This product launch would help strengthen the company's product portfolio for transcatheter products, enabling the Abbott to account for the majority share of the global market over the next few years.
Based on the application segment, the market is bifurcated into Cardiovascular, Oncology, Neurology, Urology and Others. With a dominant revenue share, cardiovascular is the largest application category in 2023. It is projected that increased patient pool of fatal cardiac disorders such as mitral regurgitation, aortic stenosis, and tricuspid regurgitation across the globe would fuel segment development. Another aspect driving the use of transcatheter devices in cardiovascular space is progress in cardiac complications along with substantial mortality decreases with the use of the new fourth-generation TAVR systems.
Transcathetar Devices Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Transcathetar Devices Market |
| Market Size in 2023 | USD 1198.34 Million |
| Market Forecast in 2032 | USD 2830.24 Million |
| Growth Rate | CAGR of 10.02% |
| Number of Pages | 130 |
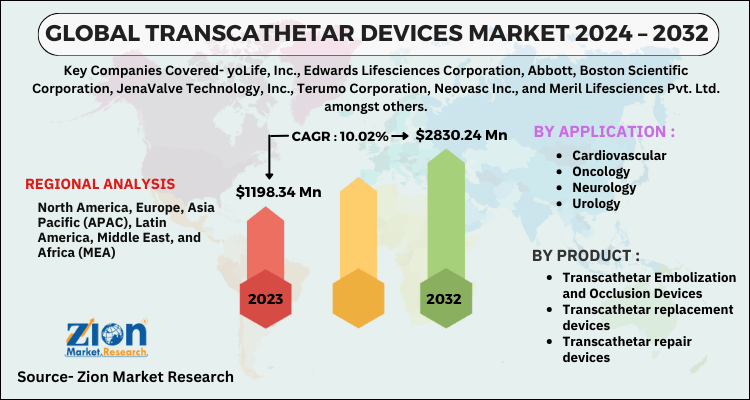
| Key Companies Covered | yoLife, Inc., Edwards Lifesciences Corporation, Abbott, Boston Scientific Corporation, JenaValve Technology, Inc., Terumo Corporation, Neovasc Inc., and Meril Lifesciences Pvt. Ltd. amongst others |
| Segments Covered | By product, By application and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Transcathetar Devices Market: Regional Analysis
The geographical segmentation includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America, and Middle East & Africa. North America accounted for the largest share of revenue on the market for transcatheter products in 2023. Increasing geriatric population, increased prevalence of valvular diseases in the geriatric community, increased spending on health care, and increased demand for minimally invasive surgery are some of the major drivers of market growth. The number of transcatheter valve replacement procedures in this population is increasing due to the growing geriatric population at high risk of open surgery. In addition, growing preference for transcatheter replacement or repair procedures over open cardiac surgery, combined with favorable government initiatives and reimbursement policies, are some of the key factors responsible for the dominant revenue share.
Transcathetar Devices Market: Competitive Players
Key players within global Transcathetar Devices market include:
- CryoLife Inc.
- Edwards Lifesciences Corporation
- Abbott
- Boston Scientific Corporation
- JenaValve Technology Inc.
- Terumo Corporation
- Neovasc Inc.
- Meril Lifesciences Pvt. Ltd.
The Global Transcathetar Devices Market is segmented as follows:
Global Transcathetar Devices Market: Product Segment Analysis
- Transcathetar Embolization and Occlusion Devices
- Transcathetar replacement devices
- Transcathetar repair devices
Global Transcathetar Devices Market: Application Segment Analysis
- Cardiovascular
- Oncology
- Neurology
- Urology
- Others
Global Transcathetar Devices Market: Regional Segment Analysis
- North America
- U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Transcatheter devices are medical devices used in minimally invasive procedures to diagnose or treat various cardiovascular conditions.
According to study, the Transcathetar Devices Market size was worth around USD 1198.34 million in 2023 and is predicted to grow to around USD 2830.24 million by 2032.
The CAGR value of Transcathetar Devices Market is expected to be around 10.02% during 2024-2032.
North America has been leading the Transcathetar Devices Market and is anticipated to continue on the dominant position in the years to come.
The Transcathetar Devices Market is led by players like yoLife Inc., Edwards Lifesciences Corporation, Abbott, Boston Scientific Corporation, JenaValve Technology, Inc., Terumo Corporation, Neovasc Inc., and Meril Lifesciences Pvt. Ltd. amongst others.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed