Omics-Based Clinical Trials Market Size, Share, Trends, Growth 2034

Omics-Based Clinical Trials Market By Phase Type (Phase 1, Phase II, Phase III, and Phase IV), By Study Design Type (Observational Studies, Interventional Studies, and Expanded Access Studies), By Indication Type (Genetic Diseases, Respiratory Diseases, Oncology, Immunology, CNS Diseases, Skin Diseases, and Others), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 6.46 Billion | USD 13.56 Billion | 7.70% | 2024 |
Omics-Based Clinical Trials Industry Perspective:
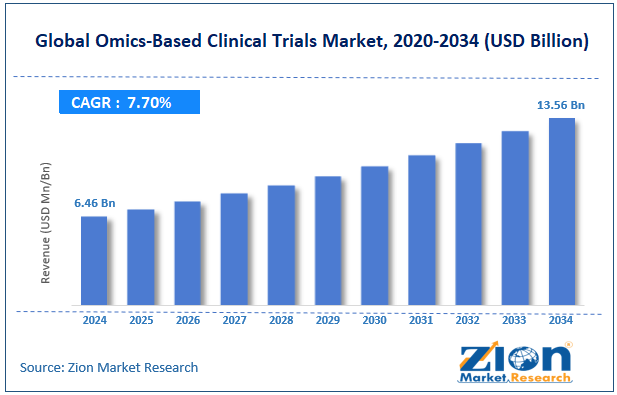
The global omics-based clinical trials market size was worth around USD 6.46 billion in 2024 and is predicted to grow to around USD 13.56 billion by 2034, with a compound annual growth rate (CAGR) of roughly 7.70% between 2025 and 2034.
Omics-Based Clinical Trials Market: Overview
Omics-based clinical trials aim to deliver highly personalized medical treatment by studying a patient’s proteomics, transcriptomics, and genomics. Clinical trials are designed to analyze several biological components of a patient to develop more customized treatments that produce effective results.
According to market research, at present, the industry for omics-based clinical trials revolves around chronic diseases such as cardiovascular conditions, oncology, and rare medical disorders.
Omics-based technologies identify biomarkers that are associated with specific medical conditions, helping pharmaceutical and biopharmaceutical companies develop effective drugs, treatment plans, and diagnostic strategies.
In addition, studying and analyzing omics can assist in developing new therapies and improving the efficiency of the current healthcare infrastructure. During the forecast period, the global omics-based clinical trials industry is projected to grow due to the rising demand for efficient cancer care around the globe.
In addition, innovation in terms of ancillary technologies helping drug developers achieve accurate results may open new avenues for further growth. One of the key growth restraints in the industry is the high cost associated with clinical trials and exhaustive regulatory challenges.
Key Insights:
- As per the analysis shared by our research analyst, the global omics-based clinical trials market is estimated to grow annually at a CAGR of around 7.70% over the forecast period (2025-2034)
- In terms of revenue, the global omics-based clinical trials market size was valued at around USD 6.46 billion in 2024 and is projected to reach USD 13.56 billion by 2034.
- The omics-based clinical trials market is projected to grow at a significant rate due to the rising demand for improved cancer detection and treatment strategies.
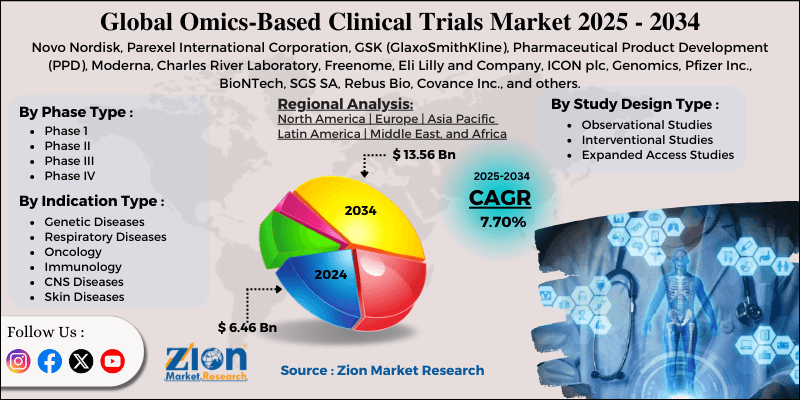
- Based on the phase type, the Phase III segment is growing at a high rate and will continue to dominate the global market as per industry projections.
- Based on the indication type, the oncology segment is anticipated to command the largest market share.
- Based on region, North America is projected to dominate the global market during the forecast period.
Omics-Based Clinical Trials Market: Growth Drivers
Rising demand for improved cancer detection and treatment strategies to fuel market revenue
The global omics-based clinical trials market is projected to grow due to the rising demand for improved cancer detection solutions and treatment strategies.
According to several official healthcare agencies and global agencies, cancer is one of the leading causes of medically-caused deaths worldwide. Cleveland Clinic suggests that currently there are more than 100 types of cancers affecting people of all age groups and genders.
Current methods of cancer diagnosis and treatment are ineffective and reduce the worrying fatality rate caused by the disease. In most cases, cancer, if detected in early stages, can be cured with efficient treatment programs.
However, several types of cancer cannot be detected in the early stages, putting the lives of patients at risk. These factors have led to an increased demand for novel cancer early-detection technologies.
Furthermore, personalized treatment programs that depend on the genomics and biological characteristics of the patient have higher chances of targeting cancer cells than standard treatment procedures. Key market players are focusing on leveraging the offerings of Artificial Intelligence (AI) and multi-omics to develop novel cancer drugs.
Increasing funding and participation from drug developers for omics-based treatment development to prove beneficial
Omic-based treatments have shown extraordinary results in handling rare conditions and severe chronic diseases. These success stories have led to increased funding backing more research and clinical trials powered by omics-based analysis of metabolomic, genomic, transcriptomic, and proteomic data.
For instance, in October 2024, Macrogen and PacBio announced the launch of a new facility aiming to improve genomics breakthrough development in Singapore. The partnership is expected to pave the way for translating genomic discoveries into more commercial solutions.
The global omic-based clinical trials market will be influenced by the launch of several government-aided favorable policies for conducting new clinical trials, which will have long-lasting impacts on the global healthcare infrastructure.
Omics-Based Clinical Trials Market: Restraints
Expenses associated with an initial investment to limit the industry’s expansion rate
The global omics-based clinical trials industry is expected to be restricted by the high expenses associated with conducting clinical research & development using biological components of patients.
The use of next-generation equipment, such as mass spectrometers, along with the cost incurred in employing skilled talent, affects the overall investments reported in the market.
Furthermore, omics-based clinical trials can be conducted only under technologically advanced facilities, which also impacts the overall revenue of the market.
Omics-Based Clinical Trials Market: Opportunities
Ongoing innovations in terms of new equipment and technologies aiding clinical trials to create growth opportunities
The global omics-based clinical trials market is projected to generate growth opportunities due to the increasing rate of innovation in the industry. For instance, the growing inclination toward the use of Artificial Intelligence and Machine Learning (ML) to obtain and analyze data while identifying key biomarkers can open new avenues for further growth for market players.
For instance, in February 2025, Illumina, Inc., a leading biotechnology company, announced the launch of an extensive series of novel innovations as the company has successfully managed to establish the largest portfolio by any company in omics solutions and sequencing applications.
Illumina has launched solutions ranging from spatial transcriptomics, genomics, epigenetics, single cell analysis, Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) technologies, and data analytics software. These solutions are expected to help drug-making companies gain revolutionary insights into key causes of diseases.
In February 2025, Compugen Ltd, an Israel-based clinical-stage cancer immunotherapy company, announced the expansion of the offering of the company’s AI/ML computational discovery platform,
Unigen. The platform will now offer high-throughput single-cell sequencing and will be used to uncover new data on gene structure for immuno-oncology.
Omics-Based Clinical Trials Market: Challenges
Strict regulatory measures worldwide to impede market expansion trajectory
The global omics-based clinical trials industry is expected to be challenged by the presence of strict regional regulations and laws governing clinical trials on omics-based biomolecules.
In addition, the lack of standard protocols for conducting omics-based clinical trials or using the output for drug development can further impede the industry’s growth rate.
Omics-Based Clinical Trials Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Omics-Based Clinical Trials Market |
| Market Size in 2024 | USD 6.46 Billion |
| Market Forecast in 2034 | USD 13.56 Billion |
| Growth Rate | CAGR of 7.70% |
| Number of Pages | 211 |
| Key Companies Covered | Novo Nordisk, Parexel International Corporation, GSK (GlaxoSmithKline), Pharmaceutical Product Development (PPD), Moderna, Charles River Laboratory, Freenome, Eli Lilly and Company, ICON plc, Genomics, Pfizer Inc., BioNTech, SGS SA, Rebus Bio, Covance Inc., and others. |
| Segments Covered | By Phase Type, By Study Design Type, By Indication Type, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Omics-Based Clinical Trials Market: Segmentation
The global omics-based clinical trials market is segmented based on phase type, study design type, indication type, and region.
Based on the phase type, the global market segments are Phase 1, Phase II, Phase III, and Phase IV. In 2024, the highest revenue was generated by the Phase III segment, dominating nearly 55% of the overall revenue. Market research suggests a growing number of phase III trials involving a larger number of participants suffering from different types of diseases.
During the forecast period, phase I is expected to deliver the highest CAGR, led by increasing studies toward developing new drugs and treatment programs.
Based on design type, the omics-based clinical trials industry divisions are observational studies, interventional studies, and expanded access studies.
Based on the indication type, the global market divisions are genetic diseases, respiratory diseases, oncology, immunology, CNS diseases, skin diseases, and others. In 2024, the highest growth was listed in the oncology segment. The increasing number of deaths caused by cancer worldwide and the lack of effective treatments have created higher segmental demand.
According to the World Health Organization, more than 10 million people died due to cancer in 2020. The growing focus on early cancer detection may provoke higher segmental revenue in the coming years.
Omics-Based Clinical Trials Market: Regional Analysis
North America to lead the market during the forecast period, according to projections
The global omics-based clinical trials market is projected to be led by North America during the forecast period. In 2024, it dominated nearly 38.05% of the global revenue, with the US taking the lead in the regional industry. The growing awareness around the benefits of early cancer detection and a research-oriented healthcare industry will fuel regional revenue in the coming years.
In January 2025, an American multinational technology company announced a novel partnership with Illumina for applying AI and genomic technologies for analyzing and interpreting multi-omic data in drug discovery, human health, and clinical research.
In February 2025, the global healthcare and clinical research sectors witnessed the launch of Evo 2, a highly powerful foundation model that assists in understanding the genetic code for all living organisms.
The AI model for genomic data is powered by the NVIDIA DGX Cloud platform in partnership with Stanford University and the nonprofit biomedical research organization Arc Institute. The presence of a robust regional framework governing clinical trials based on omics will further aid higher revenue in North America.
Omics-Based Clinical Trials Market: Competitive Analysis
The global omics-based clinical trials market is led by players like:
- Novo Nordisk
- Parexel International Corporation
- GSK (GlaxoSmithKline)
- Pharmaceutical Product Development (PPD)
- Moderna
- Charles River Laboratory
- Freenome
- Eli Lilly and Company
- ICON plc
- Genomics
- Pfizer Inc.
- BioNTech
- SGS SA
- Rebus Bio
- Covance Inc.
The global omics-based clinical trials market is segmented as follows:
By Phase Type
- Phase 1
- Phase II
- Phase III
- Phase IV
By Study Design Type
- Observational Studies
- Interventional Studies
- Expanded Access Studies
By Indication Type
- Genetic Diseases
- Respiratory Diseases
- Oncology
- Immunology
- CNS Diseases
- Skin Diseases
- Others
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Omics-based clinical trials aim to deliver highly personalized medical treatment by studying a patient’s proteomics, transcriptomics, and genomics.
The global omics-based clinical trials market is projected to grow due to the rising demand for improved cancer detection solutions and treatment strategies.
According to study, the global omics-based clinical trials market size was worth around USD 6.46 billion in 2024 and is predicted to grow to around USD 13.56 billion by 2034.
The CAGR value of the omics-based clinical trials market is expected to be around 7.70% during 2025-2034.
The global omics-based clinical trials market is projected to be led by North America during the forecast period.
The global omics-based clinical trials market is led by players like Novo Nordisk, Parexel International Corporation, GSK (GlaxoSmithKline), Pharmaceutical Product Development (PPD), Moderna, Charles River Laboratory, Freenome, Eli Lilly and Company, ICON plc, Genomics, Pfizer Inc., BioNTech, SGS SA, Rebus Bio, and Covance Inc.
The report explores crucial aspects of the omics-based clinical trials market, including a detailed discussion of existing growth factors and restraints, while browsing future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed