Non-Invasive Prenatal Testing (NIPT) Market Size, Share, Growth Report 2032

Non-Invasive Prenatal Testing (NIPT) Market By Test Type (Panel 1, Panel 2 And Panel 3) And End-Users (Hospitals, Diagnostic Centers And Maternity Clinics): Global Industry Perspective, Comprehensive Analysis And Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 6.29 Billion | USD 19.21 Billion | 13.2% | 2023 |
Non-Invasive Prenatal Testing (NIPT) Market Insights
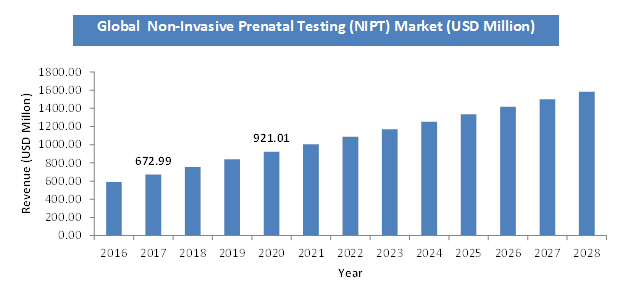
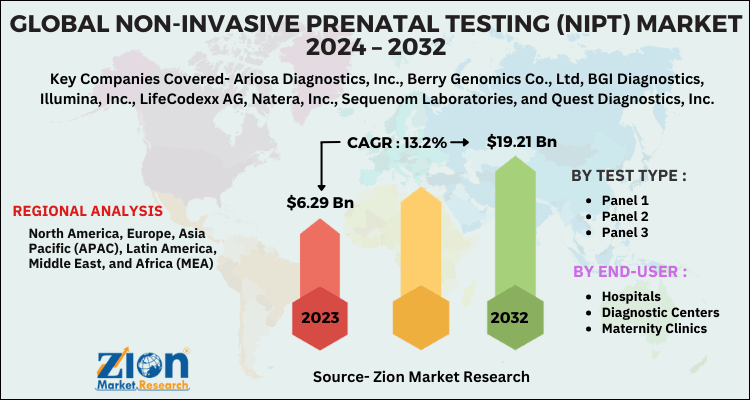
According to a report from Zion Market Research, the global Non-Invasive Prenatal Testing (NIPT) Market was valued at USD 6.29 Billion in 2023 and is projected to hit USD 19.21 Billion by 2032, with a compound annual growth rate (CAGR) of 13.2% during the forecast period 2024-2032. This report explores market strengths, weakness, opportunities, and threats. It also provides valuable insights into the market's growth drivers, challenges, and the future prospects that may emerge in the Non-Invasive Prenatal Testing (NIPT) Market industry over the next decade.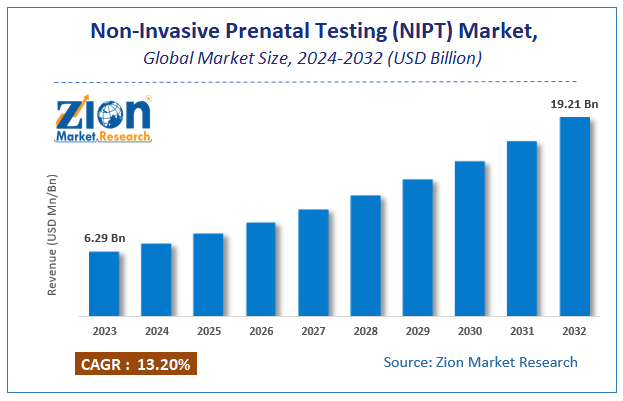 Request Free Sample
Request Free Sample
Market Overview
Non-invasive prenatal testing is a complicated technology that helps determine fetal chromosomal anomalies by analyzing the fetus's cell-free DNA. because the invasive procedures have a better risk quotient, including the danger of fetal loss, most healthcare professionals prefer non-invasive techniques. NIPTs are new genetic tests that use cell-free circulating fetal DNA within the maternal serum to screen for more common fetal aneuploidies, like mongolism, trisomy 18 (Edward syndrome), trisomy 13 (Patau syndrome), and monosomy X (Turner syndrome). With increased patient access to healthcare information also as advertising, the NIPT market is anticipated to possess a positive outlook within the coming years.
Prenatal screening and diagnosis involve testing for conditions in a fetus before it is born, with the aim of detecting causes of birth defects, abnormalities, and genetic conditions. Advancing the prenatal care services provided across the globe has become an uphill battle for medical practitioners and drug makers. Fortunately, this struggle is being eased by the active participation of parents from all around the world in protecting the health of their soon-to-be-born babies through prenatal disease detection methods. Noninvasive prenatal testing (NIPT) procedures are, hence, being rapidly adopted, which is prompting healthcare service providers to increase their focus on such prenatal diagnosis & screening services. Application of the technique to analyze approximately 20,000 genetic features in a single reaction in a sensitive and accurate manner for detection of a wide range of genetic disorders with fewer false positives is expected to propel the demand for NIPT products in the coming years.
The vendors are increasingly entering into partnerships and collaborations to extend their product offerings, increase their market shares, and expand their operations. These partnerships and collaborations help vendors enhance their R&D capabilities for manufacturing innovative devices that meet consumer needs. For instance, in 2015, Human Longevity entered into collaboration with Roche Diagnostics to conduct whole-genome sequencing of Roche Diagnostics' subsidiary Genentech's de-identified samples. The partnership helped Roche Diagnostics analyze patient samples based on precise genetic categories and discover new diagnostics and targeted therapies. These partnerships and collaborative activities are accelerating drug discovery efforts, which will lead to the development of new therapies to detect chromosomal abnormalities in neonates. To increase the adoption of new non-invasive tests, many companies focus on developing and strengthening their research infrastructure to develop efficient NIPTs. The competitive environment in this market is expected to intensify with an increase in product/service extensions, technological innovations, and mergers and acquisitions.
Growth Factors
Advantages offered by NIPT like non-invasiveness, high accuracy, and early detection, raising awareness, increasing penetration in highly untapped countries in Asia and Latin America, and continuous increase in average maternal age are the main factors expected to drive the expansion of the worldwide NIPT market during the forecast period. However, the market is additionally facing significant ethical and regulatory hurdles related to the implementation of NIPT due to the assumption that it's likely to extend the incidence of abortions. Therefore, various professional organizations like the American College of Obstetricians and Gynecologists (ACOG), the International Society for a diagnostic procedure, the Japan Society of Obstetrics and Gynecology, and genetic counselors across the planet have found out guidelines that limit the utilization of non-invasive prenatal testing only to pregnant women at high risk of chromosomal aneuploidies.
The global NIPT market is highly fragmented owing to the presence of several well-established global and regional vendors. The majority of vendors are looking to expand their businesses in the APAC region, which is a major untapped market that is likely to grow at a rapid pace during the forecast period. To increase the adoption of new non-invasive tests, many companies focus on developing and strengthening their research infrastructure to develop efficient NIPTs. The competitive environment in this market is expected to intensify with an increase in product/service extensions, technological innovations, and mergers and acquisitions.
Non-Invasive Prenatal Testing (NIPT) Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Non-Invasive Prenatal Testing (NIPT) Market |
| Market Size in 2023 | USD 6.29 Billion |
| Market Forecast in 2032 | USD 19.21 Billion |
| Growth Rate | CAGR of 13.2% |
| Number of Pages | 130 |
| Key Companies Covered | Ariosa Diagnostics, Inc., Berry Genomics Co., Ltd, BGI Diagnostics, Illumina, Inc., LifeCodexx AG, Natera, Inc., Sequenom Laboratories, and Quest Diagnostics, Inc. |
| Segments Covered | By Test Type, By End-User and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Segment Analysis Preview
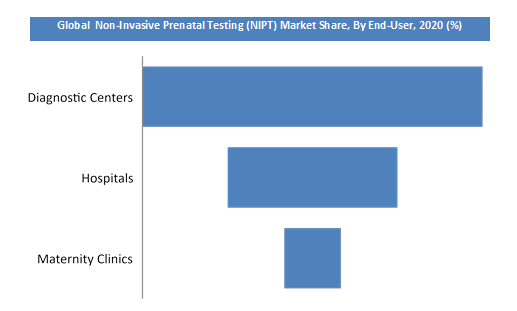
Based on test type the market is segmented into panel 1, panel 2 and panel 3. Based on end-user, the worldwide marketplace for OR equipment is segmented into hospitals, diagnostic centers, and maternity clinics. Diagnostic centers have accounted for the main share of this segment in 2018 due to the increased investments in healthcare infrastructure & the use of advanced technologies. The increasing incidence of chromosomal abnormalities coupled with growing product usage in new applications is driving the organic revenue growth in the market. Additionally, increasing demand for patient satisfaction, cost-effective procedures, and efficient practices by healthcare professionals also are expected to spice up the expansion of this market within the forecast period.
Regional Analysis Preview
Based on geography, North America held the most important share of the worldwide NIPT market in terms of revenue. Major players domiciled within the U.S. and comparatively high awareness levels about NIPT in North America are the key factors attributed to the region’s leadership position. In Europe, market growth largely depends upon the increasing penetration of those U.S.-based test developers and an increase in acceptance of those tests. Japan, Australia, China, and India represent high growth potential markets in Asia-Pacific. The NIPT market in China is dominated by two local players BGI Diagnostics and Berry Genomics. Additionally, the recent CFDA approval for Bambini Test would propel the expansion of the market during this region.
Key Market Players & Competitive Landscape
The major players that are comprised in Non-Invasive Prenatal Testing (NIPT) market are :
- Ariosa Diagnostics, Inc.
- Berry Genomics Co., Ltd
- BGI Diagnostics
- Illumina, Inc.
- LifeCodexx AG
- Natera, Inc.
- Sequenom Laboratories
- Quest Diagnostics, Inc.
The market is saturated with few players operating in the NIPT space and these companies are expected to compete on the grounds of cost and functionality. Product differentiation, new product launches and merger and acquisition are some of the strategies adopted by these industry participants.
The global Non-Invasive Prenatal Testing (NIPT) Market is segmented as follows:
By Test Type
- Panel 1
- Panel 2
- Panel 3
By End-User
- Hospitals
- Diagnostic Centers
- Maternity Clinics
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
The global Non-Invasive Prenatal Testing (NIPT) Market was valued at USD 6.29 Billion in 2023.
The global Non-Invasive Prenatal Testing (NIPT) Market is expected to reach USD 19.21 Billion by 2032, with a CAGR of around 13.2% between 2024-2032.
The increasing incidence of chromosomal abnormalities coupled with growing product usage in new applications is driving the organic revenue growth in the market.
North America in 2023 ruled the Non-Invasive Prenatal Testing (NIPT) market and was believed to be the highest income-generating area all over the globe.
The major players that are comprised in Non-Invasive Prenatal Testing (NIPT) market are Ariosa Diagnostics, Inc., Berry Genomics Co., Ltd, BGI Diagnostics, Illumina, Inc., LifeCodexx AG, Natera, Inc., Sequenom Laboratories, and Quest Diagnostics, Inc.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed