Global Medical Devices Vigilance Market Size, Share, Growth Analysis Report - Forecast 2034

Medical Devices Vigilance Market By Delivery Mode (On-premise, Cloud-based), By Application (Post-Market Surveillance, Compliance Management, Risk Management, Audit & Inspection, Case Management), By End-user (Manufacturers, Regulatory Authorities, Hospitals, Contract Research Organizations (CROs)), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 7.93 Billion | USD 17.43 Billion | 8.2% | 2024 |
Medical Devices Vigilance Market: Industry Perspective
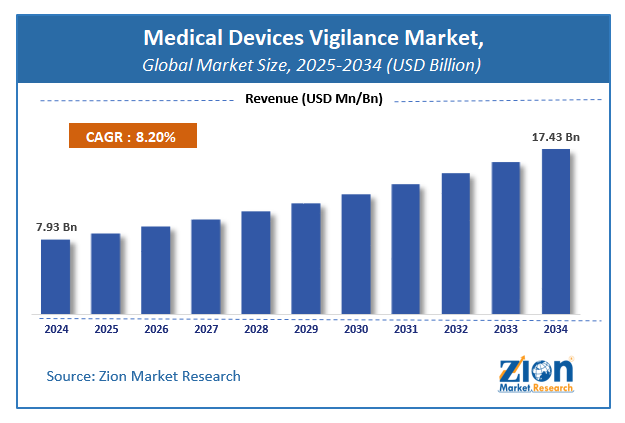
According to the report, global demand for Medical Devices Vigilance market was worth around USD 7.93 Billion in 2024 and is predicted to grow to around USD 17.43 Billion by 2034 with a compound annual growth rate (CAGR) of roughly 8.2% between 2025 and 2034. The report analyzes the global medical devices vigilance market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the medical devices vigilance industry.
Global Medical Devices Vigilance Market: Overview
With the testing of medical equipment becoming necessary for the healthcare industry, medical device vigilance is gaining prominence across the globe. The recognition of potential hazards pertaining to medical instruments is included in medical device vigilance. The vigilance market for medical devices can be traced back to 1992, when the Global Harmonization Task Force (GHTF) was formed to standardize national regulatory frameworks for medical devices. The goal was to improve access to effective, secure and clinically useful medical technologies.
Key Insights
- As per the analysis shared by our research analyst, the global medical devices vigilance market is estimated to grow annually at a CAGR of around 8.2% over the forecast period (2025-2034).
- Regarding revenue, the global medical devices vigilance market size was valued at around USD 7.93 Billion in 2024 and is projected to reach USD 17.43 Billion by 2034.
- The medical devices vigilance market is projected to grow at a significant rate due to regulatory focus on patient safety, increasing device complexity, and the need for real-time monitoring and reporting of adverse events.
- Based on Delivery Mode, the On-premise segment is expected to lead the global market.
- On the basis of Application, the Post-Market Surveillance segment is growing at a high rate and will continue to dominate the global market.
- Based on the End-user, the Manufacturers segment is projected to swipe the largest market share.
- Based on region, North America is predicted to dominate the global market during the forecast period.
Global Medical Devices Vigilance Market: Growth Factors
The surge in the number of medical devices recalls due to safety issues is the key factor driving the global market growth during the forecast period. Additionally, growing awareness among people pertaining to availability of medical device vigilance software and reporting of adverse events will positively leverage medical devices vigilance market growth over the analysis timeline.
Furthermore, favorable government scenario pertaining to medical devices vigilance ensuing patient safety will serve as an impact rendering factor that will bolster the business scope. Increasing burden on medical devices manufacturers to produce safe medical equipment as well as strict safety laws put into practice/use by regulatory bodies pertaining to pre- and post-commercialization of medical equipment will further spur the market revenue. However, carelessness of production units towards the maintenance of product safety may refrain the growth of medical devices vigilance market over the upcoming years.
Global Medical Devices Vigilance Market: Segmentation
The global medical devices vigilance market can be classified into the delivery mode, application, and end-user.
Based on the delivery mode, the market is sectored into on-demand and on-premise.
On the basis of application, the market is segmented into diagnostic, surgical, therapeutic, and research sectors.
Based on the end-user, the medical devices vigilance market is classified into clinical research organizations, original equipment manufacturers, and business process outsourcing.
Global Medical Devices Vigilance Market: Regional Analysis
Based on the region, the global medical devices vigilance market can be divided into five main regions: North America, Latin America, Europe, Asia Pacific, and the Middle East and Africa. North America is likely to dominate the global medical devices vigilance market growth over the years ahead, owing to the tremendous demand for medical devices vigilance in the region. A massive number of reported hostile activities will elevate the acceptance of vigilance systems. A strong base of medical equipment producing firms in North America will further augment regional business growth. Moreover, Asia Pacific medical devices vigilance market will register a profitable growth over the years to come, owing to the growing focus of medical device manufacturers on product safety. Additionally, the presence of a large and diverse patient pool in the region coupled with a surge in clinical research outsourcing will further accelerate regional market growth.
Medical Devices Vigilance Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Medical Devices Vigilance Market |
| Market Size in 2024 | USD 7.93 Billion |
| Market Forecast in 2034 | USD 17.43 Billion |
| Growth Rate | CAGR of 8.2% |
| Number of Pages | 110 |
| Key Companies Covered | PZEINCRO, Sparta Systems, AssurX, Oracle, Xybion, INTEL, Sarjen Systems, MDI Consultants, AB-Cube, Numerix, and Omnify Software., and others. |
| Segments Covered | By Delivery Mode, By Application, By End-user, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
To know more about this report, request a sample copy.
The medical devices vigilance market in Europe is slated to experience rapid growth, subject to stringent medical device regulations and policies in countries such as the UK. Latin America and the Middle East and Africa possess brighter market growth prospects and are likely to contribute considerably towards the global market share in the upcoming years.
Medical Devices Vigilance Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the medical devices vigilance market on a global and regional basis.
The global medical devices vigilance market is dominated by players like:
- PZEINCRO
- Sparta Systems
- AssurX
- Oracle
- Xybion
- INTEL
- Sarjen Systems
- MDI Consultants
- AB-Cube
- Numerix
- and Omnify Software.
The global medical devices vigilance market is segmented as follows;
By Delivery Mode
- On-premise
- Cloud-based
By Application
- Post-Market Surveillance
- Compliance Management
- Risk Management
- Audit & Inspection
- Case Management
By End-user
- Manufacturers
- Regulatory Authorities
- Hospitals
- Contract Research Organizations (CROs)
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
The global medical devices vigilance market is expected to grow due to regulatory focus on patient safety, increasing device complexity, and the need for real-time monitoring and reporting of adverse events.
According to a study, the global medical devices vigilance market size was worth around USD 7.93 Billion in 2024 and is expected to reach USD 17.43 Billion by 2034.
The global medical devices vigilance market is expected to grow at a CAGR of 8.2% during the forecast period.
North America is expected to dominate the medical devices vigilance market over the forecast period.
Leading players in the global medical devices vigilance market include PZEINCRO, Sparta Systems, AssurX, Oracle, Xybion, INTEL, Sarjen Systems, MDI Consultants, AB-Cube, Numerix, and Omnify Software., among others.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed