Enbrel Market Size, Share, Growth, Global Trends, Forecast 2034

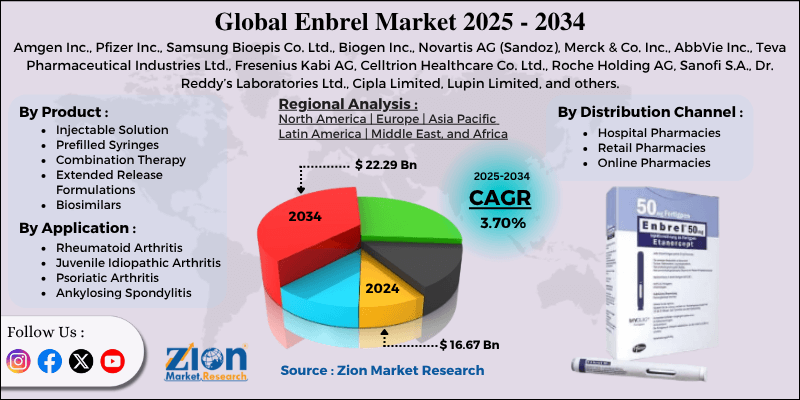
Enbrel Market By Product (Injectable Solution, Prefilled Syringes, Combination Therapy, Extended Release Formulations, Biosimilars), By Application (Rheumatoid Arthritis, Juvenile Idiopathic Arthritis, Psoriatic Arthritis, Ankylosing Spondylitis, and Others), By Distribution Channel (Hospital Pharmacies, Retail Pharmacies, Online Pharmacies), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 16.67 Billion | USD 22.29 Billion | 3.70% | 2024 |
Enbrel Industry Perspective:
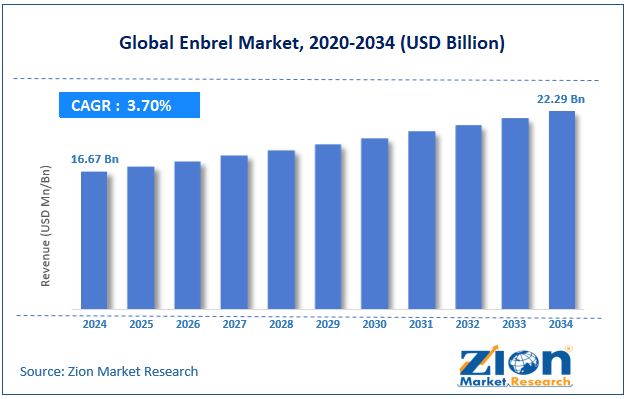
The global Enbrel market size was approximately USD 16.67 billion in 2024 and is projected to reach around USD 22.29 billion by 2034, with a compound annual growth rate (CAGR) of approximately 3.70% between 2025 and 2034.
Key Insights:
- As per the analysis shared by our research analyst, the global Enbrel market is estimated to grow annually at a CAGR of around 3.70% over the forecast period (2025-2034)
- In terms of revenue, the global Enbrel market size was valued at around USD 16.67 billion in 2024 and is projected to reach USD 22.29 billion by 2034.
- The Enbrel market is projected to grow significantly owing to the rising geriatric population, growth of healthcare infrastructure, and advancements in biotechnology.
- Based on product, the prefilled syringes segment is expected to lead the market, while the injectable solution segment is expected to grow considerably.
- Based on application, the rheumatoid arthritis segment is the largest, while the psoriatic arthritis segment is projected to experience substantial revenue growth over the forecast period.
- Based on the distribution channel, the hospital pharmacies segment is expected to lead the market, followed by the retail pharmacies segment.
- Based on region, North America is projected to dominate the global market during the estimated period, followed by Europe.
Enbrel Market: Overview
Enbrel, also known as etanercept, is a biologic medication used to treat autoimmune disorders, including psoriatic arthritis, rheumatoid arthritis, plaque psoriasis, and ankylosing spondylitis. It works by preventing tumor necrosis factor (TNF), a substance in the body that causes inflammation and contributes to overactivity of the immune system. The global Enbrel market is expected to expand rapidly, driven by the aging global population, increased awareness and early diagnosis, as well as a strong clinical efficacy and safety profile. Older adults are more vulnerable to chronic degenerative joint and inflammatory diseases. With the global population aged 60 and above projected to double by 2050, the demand for biologics like Enbrel is poised to increase. This demographic assures a steady patient pool needing long-term therapy.
Moreover, enhanced public awareness campaigns and diagnostic capabilities have led to the early detection of autoimmune conditions. Early interventions with biologics like Enbrel can help prevent disease progression, driving higher prescription rates. Enbrel's long-standing track record of efficacy and safety makes it a preferred treatment choice among healthcare providers. Clinical studies consistently present its ability to reduce inflammation, progression, and joint pain in rheumatoid arthritis. This reliability backs patient adherence and physician confidence.
Despite the growth, the global market is impeded by factors such as the high cost of treatment and biosimilar competition. Biologic therapies like Enbrel are high-priced, restricting accessibility in the middle- and low-income nations. Annual treatment costs may range from USD 15,000 to USD 30,000, placing a significant burden on patients without proper insurance. Cost concerns remain a significant challenge to broader adoption. Similarly, the patent expiration of Enbrel has led to the growth of biosimilars, such as Erelzi and Benepali. These cost-effective alternatives are corroding industry share in Asia and Europe. Elevated price competition pressures profitability and revenue for the originator brand.
Nonetheless, the global Enbrel industry stands to gain from several key opportunities, including the development of next-generation formulations and a rising focus on personalized medicine. Innovating enhanced Enbrel formulations, such as combination therapies or extended-release versions, may improve industry appeal. Next-generation delivery systems may enhance compliance and reduce the frequency of dosing. These innovations offer differentiation in a competitive landscape. The rising adoption of precision medicine can optimize Enbrel's use, depending on genetic profiling. Tailored treatment approaches enhance therapeutic outcomes and patient satisfaction. This trend supports the healthcare industry's shift towards individualized care models.
Enbrel Market Dynamics
Growth Drivers
How are patent expirations and biosimilar competition driving growth in the Enbrel market?
The launch of etanercept biosimilars worldwide is transforming industry dynamics, creating both increased patient access and price pressure. Competitive pricing, tendering programs, and mandating substitution policies are driving the adoption of biosimilars in many regions. While the originator of Enbrel experiences volume erosion, it continues to benefit from physician preference and brand recognition in specific markets. The degree of impact varies by geography, with Europe witnessing faster penetration than the United States. Hence, biosimilar competition is a key propeller shaping sales tactics and pricing models in the global Enbrel market.
How is the Enbrel market fueled by patient support, adherence programs, and delivery innovation?
Patient adherence and convenience play a vital role in maintaining a substantial market share for Enbrel, with innovations in autoinjectors and prefilled syringes improving usability. Support programs providing home delivery, training, and cost assistance motivate treatment persistence. These services reduce the need for switching to biosimilars and enhance patient outcomes. Digital tools and adherence monitoring further boost engagement. Consequently, patient-centric initiatives fuel sustained utilization and industry loyalty.
Restraints
Approval delays and strict regulatory requirements negatively impact the market progress
Regulatory scrutiny for biologics remains strict, with approval processes in developing regions often delayed due to the complex requirements for pharmacovigilance and clinical data. Even minor manufacturing changes require regulatory notification, creating a complex chain of barriers. These regulatory challenges may slow the launch of novel formulations, delivery services, or indications. Recent reports indicate delays in several Latin American and Asian regions due to local trial requirements. These regulatory complexities limit industry expansion and restrict patient access to treatment.
Opportunities
How do technological innovations in drug delivery offer advantageous conditions for the Enbrel market development?
Advancements like prefilled syringes, autoinjectors, and digital adherence tools enhance patient adherence and convenience. Enhanced delivery systems reduce injection-site pain and errors, motivating longer treatment persistence. The launch of enhanced Enbrel devices in Europe and the United States has resulted in a 15% increase in patient satisfaction rates, as indicated in surveys. These advancements may distinguish Enbrel from several NF inhibitors and biosimilars. The adoption of patient-friendly devices represents a tangible opportunity to increase lifetime treatment value, positively impacting the growth of the Enbrel industry.
Challenges
Supply chain and manufacturing complexities restrict the market growth
Biologics need complex manufacturing, stringent quality control, and cold-chain logistics. Any disturbance in distribution or production may lead to shortages, lost revenue, and delayed treatments. The worldwide pandemic underscored vulnerabilities in biologic supply chains, impacting timely delivery. Regulatory audits and compliance needs further complicate operations. Maintaining a consistent supply while managing costs remains a significant challenge for the global market.
Enbrel Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Enbrel Market |
| Market Size in 2024 | USD 16.67 Billion |
| Market Forecast in 2034 | USD 22.29 Billion |
| Growth Rate | CAGR of 3.70% |
| Number of Pages | 215 |
| Key Companies Covered | Amgen Inc., Pfizer Inc., Samsung Bioepis Co. Ltd., Biogen Inc., Novartis AG (Sandoz), Merck & Co. Inc., AbbVie Inc., Teva Pharmaceutical Industries Ltd., Fresenius Kabi AG, Celltrion Healthcare Co. Ltd., Roche Holding AG, Sanofi S.A., Dr. Reddy’s Laboratories Ltd., Cipla Limited, Lupin Limited, and others. |
| Segments Covered | By Product, By Application, By Distribution Channel, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2019 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Enbrel Market: Segmentation
The global Enbrel market is segmented based on product, application, distribution channel, and region.
Based on product, the global Enbrel industry is divided into injectable solutions, prefilled syringes, combination therapy, extended-release formulations, and biosimilars. The prefilled syringes segment accounted for a significant market share due to their accuracy in dosing, ease of administration, and enhanced patient convenience. They eliminate the need for manual preparation, decreasing the threat of dosing and contamination errors. The growing preference for home-based care and self-administration among patients with chronic diseases has elevated the adoption. Additionally, technological advancements in autoinjector designs and ergonomic packaging continue to drive the segment's growth.
Based on application, the global Enbrel market is segmented into rheumatoid arthritis, juvenile idiopathic arthritis, psoriatic arthritis, ankylosing spondylitis, and others. The rheumatoid arthritis segment holds a leadership position in the market due to its high prevalence worldwide and chronic nature. According to the WHO, rheumatoid arthritis affects nearly 1% of the worldwide population, fueling continuous demand for long-term biologic therapies. Enbrel's proven efficacy in reducing joint inflammation, improving mobility, and slowing disease progression has established it as a primary biologic option. The aging population and increased early diagnosis fuel the segmental dominance.
Based on distribution channel, the global market is segmented into hospital pharmacies, retail pharmacies, and online pharmacies. The hospital pharmacies segment dominated the market due to the frequent administration of drugs in hospital and clinical settings, especially during the initial treatment stage. These facilities promise proper handling, administration, and storage of biologics under professional guidance. The availability of trained healthcare providers and adherence to stringent safety protocols make hospital pharmacies the highly preferred channel. Moreover, supportive reimbursement and patient monitoring programs in hospitals strengthen their dominant share.
Enbrel Market: Regional Analysis
Why is North America outperforming other regions in the global Enbrel Market?
North America is expected to maintain its leading position in the global Enbrel market, driven by the high prevalence of autoimmune diseases, advanced healthcare infrastructure, and supportive reimbursement policies. North America holds the leading cases of autoimmune diseases worldwide, comprising psoriatic arthritis, ankylosing spondylitis, and rheumatoid arthritis. For example, nearly 1.3 million adults in the United States have rheumatoid arthritis, propelling continuous demand for biologics like Enbrel. This high disease pressure promises a significant and constant patient base, fueling the industry dominance.
Furthermore, the region boasts well-established healthcare systems with advanced treatment facilities and diagnostics. Specialty clinics and hospitals are equipped with monitoring and biologic administration systems, promising the safe use of Enbrel. This infrastructure supports higher adoption rates and effective distribution through retail pharmacies and hospitals. Strong insurance coverage and reimbursement programs in Canada and the United States reduce out-of-pocket costs for individuals. Programs like private insurance plans or Medicare cover biologic therapies, making Enbrel affordable for chronic use. This financial support directly adds to the elevated prescriptions and sustained industry growth.
Europe ranks as the second-largest region in the global Enbrel industry, thanks to its well-developed healthcare systems, government support and reimbursement policies, as well as growing awareness and early diagnosis. European countries have a robust healthcare infrastructure, featuring modernized hospitals, extensive pharmacy networks, and specialized rheumatology clinics. These facilities enable the safe administration, distribution, and monitoring of biologics. Effective healthcare delivery systems support consistent adoption of Enbrel in both Northern and Western Europe.
Additionally, several European nations offer comprehensive reimbursement schemes for biologic therapies through their national health services. For example, countries such as France, Germany, and the UK offer coverage for chronic autoimmune treatments, thereby reducing patient out-of-pocket expenditures. This financial support motivates higher prescription rates and broader industry penetration. Public health initiatives and patient advocacy programs in Europe focus on early detection and management of autoimmune diseases. Early intervention with Enbrel enhances disease outcomes and decreases long-term disability. Increased awareness among physicians and patients fuels consistent demand within the industry.
Enbrel Market: Competitive Analysis
The leading players in the global enbrel market are:
- Amgen Inc.
- Pfizer Inc.
- Samsung Bioepis Co. Ltd.
- Biogen Inc.
- Novartis AG (Sandoz)
- Merck & Co. Inc.
- AbbVie Inc.
- Teva Pharmaceutical Industries Ltd.
- Fresenius Kabi AG
- Celltrion Healthcare Co. Ltd.
- Roche Holding AG
- Sanofi S.A.
- Dr. Reddy’s Laboratories Ltd.
- Cipla Limited
- Lupin Limited
Enbrel Market: Key Market Trends
Shift toward home-based therapy:
The increasing availability of autoinjectors and prefilled syringes enables patients to self-administer Enbrel at home, reducing hospital visits and enhancing treatment adherence and convenience. Home-based therapy is especially appealing for chronic autoimmune patients looking for long-term management.
Rising adoption of biosimilars:
With the patent expiration of Embrel, biosimilars such as Erelzi and Benepali are gaining prominence, primarily in Asia and Europe. These low-cost substitutes are increasing patient access and surging overall industry competition. This trend is fueling pricing strategies and impacting the industry share of the originator.
The global Enbrel market is segmented as follows:
By Product
- Injectable Solution
- Prefilled Syringes
- Combination Therapy
- Extended Release Formulations
- Biosimilars
By Application
- Rheumatoid Arthritis
- Juvenile Idiopathic Arthritis
- Psoriatic Arthritis
- Ankylosing Spondylitis
- Others
By Distribution Channel
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Enbrel, also known as etanercept, is a biologic medication used to treat autoimmune disorders, including psoriatic arthritis, rheumatoid arthritis, plaque psoriasis, and ankylosing spondylitis. It works by preventing tumor necrosis factor (TNF), a substance in the body that causes inflammation and contributes to overactivity of the immune system.
The global Enbrel market is projected to grow due to the increasing prevalence of autoimmune diseases, the rising adoption of biosimilars, and growing research and development investments.
According to study, the global Enbrel market size was worth around USD 16.67 billion in 2024 and is predicted to grow to around USD 22.29 billion by 2034.
The CAGR value of the Enbrel market is expected to be approximately 3.70% from 2025 to 2034.
Stringent regulatory approvals for patent expirations, biologics, and cold-chain storage requirements are significant factors influencing market growth.
North America is expected to lead the global Enbrel market during the forecast period.
The key players profiled in the global enbrel market include Amgen Inc., Pfizer Inc., Samsung Bioepis Co., Ltd., Biogen Inc., Novartis AG (Sandoz), Merck & Co., Inc., AbbVie Inc., Teva Pharmaceutical Industries Ltd., Fresenius Kabi AG, Celltrion Healthcare Co., Ltd., Roche Holding AG, Sanofi S.A., Dr. Reddy’s Laboratories Ltd., Cipla Limited, and Lupin Limited.
Stakeholders should focus on patient-centric delivery solutions, biosimilar development, market expansion in emerging regions, strategic partnerships, and value-based pricing models to stay competitive in the market.
The value chain of the global Enbrel industry includes clinical trials and R&D, manufacturing, distribution, quality control, marketing, and patient support services.
The report examines key aspects of the Enbrel market, including a detailed analysis of existing growth factors and restraints, as well as an examination of future growth opportunities and challenges that will impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed