Global CRM197 Market Size, Share, Growth Analysis Report - Forecast 2034

CRM197 Market By Type (Research Grade CRM197, CGMP Grade CRM197), By Application (Meningococcal Polysaccharide Conjugate Vaccine, Pneumococcal Conjugate Vaccine), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 265.88 Million | USD 1800.57 Million | 21.08% | 2024 |
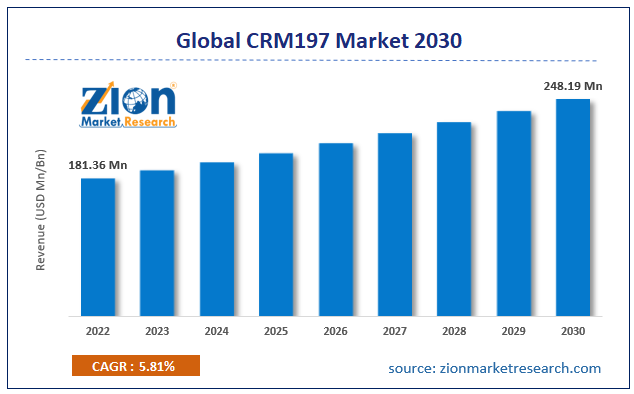
CRM197 Market: Industry Perspective
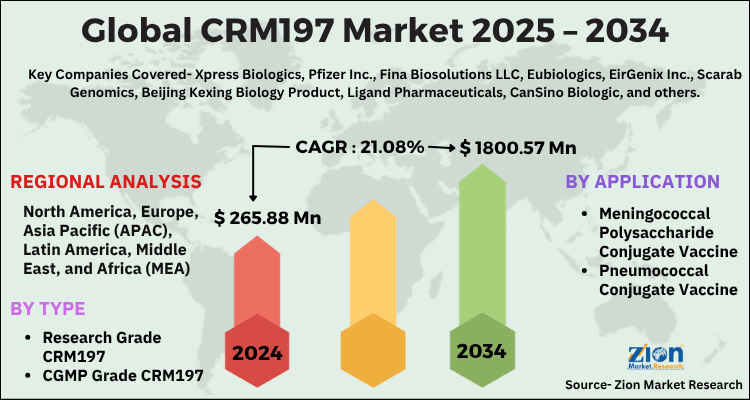
The global CRM197 market size was worth around USD 265.88 Million in 2024 and is predicted to grow to around USD 1800.57 Million by 2034 with a compound annual growth rate (CAGR) of roughly 21.08% between 2025 and 2034. The report analyzes the global CRM197 market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the CRM197 industry.
The market report is an indispensable guide on growth factors, challenges, restraints, and opportunities in the global market space. The CRM197 industry report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, PESTEL analysis, SWOT analysis, Porter’s five force analysis, and value chain analysis. Additionally, the CRM197 market report explores the investor and stakeholder space to help companies make data-driven decisions.
CRM197 Market: Overview
CRM197 is a non-toxic diphtheria toxin mutant that is used as a protein carrier for haptens and polysaccharides to make them immunogenic. Reportedly, the product is considered to be an ideal protein carrier and finds a slew of applications in approved conjugate vaccines that are used against encapsulated bacteria. Moreover, it is being assessed as a potential medicine-delivery fusion protein. In addition to this, CRM197 can also be utilized for preventing cancers having poor prognoses. It can be used in vaccines to make an individual immune to diseases such as pneumococcus, Haemophilus influenzae, and meningococcal meningitis.
Key Insights
- As per the analysis shared by our research analyst, the global CRM197 market is estimated to grow annually at a CAGR of around 21.08% over the forecast period (2025-2034).
- Regarding revenue, the global CRM197 market size was valued at around USD 265.88 Million in 2024 and is projected to reach USD 1800.57 Million by 2034.
- The CRM197 market is projected to grow at a significant rate due to increasing demand for conjugate vaccines for various infectious diseases, growing awareness and initiatives for immunization programs worldwide, advancements in vaccine research and development.
- Based on Type, the Research Grade CRM197 segment is expected to lead the global market.
- On the basis of Application, the Meningococcal Polysaccharide Conjugate Vaccine segment is growing at a high rate and will continue to dominate the global market.
- Based on region, North America is predicted to dominate the global market during the forecast period.
CRM197 Market: Dynamics
Key Growth Drivers:
CRM197 is increasingly in demand as a proven, highly immunogenic carrier protein for conjugate vaccines, driving market growth as manufacturers develop next-generation vaccines (pneumococcal, meningococcal, typhoid, and others). Rising global vaccination programs and stronger public-health initiatives—especially in pediatric immunization—boost adoption. Advances in bioprocessing and recombinant production techniques have improved yields and lowered per-dose costs, encouraging wider use. Strategic partnerships between biotech firms and vaccine manufacturers, plus growing investment in infectious-disease and oncology vaccine R&D, further propel market expansion.
Restraints:
High standards for GMP manufacturing and complex downstream purification increase production costs and create barriers for smaller manufacturers. Regulatory scrutiny around biologic carriers —including batch consistency, residual contaminants, and immunogenicity profiles—can extend development timelines and raise compliance expenses. Intellectual property/licensing constraints or long-term supplier contracts for high-quality CRM197 create supply limitations. Finally, competition from alternative carrier proteins and synthetic platforms can limit market share growth for CRM197 in certain applications.
Opportunities:
There is strong opportunity to expand CRM197 use beyond traditional pediatric conjugates into adult and therapeutic vaccines (e.g., cancer or chronic-infection vaccines) where carrier choice influences efficacy. Emerging markets increasing their immunization coverage create large, underserved demand pools. Process innovations (continuous bioprocessing, modular manufacturing) and local production partnerships offer cost and supply advantages. Co-formulation and platform approaches that standardize CRM197 conjugation could shorten development cycles and attract broader vaccine pipelines.
Challenges:
Ensuring lot-to-lot consistency in conjugation chemistry and maintaining the stability and activity of CRM197 during formulation and storage remain technical hurdles. Robust supply-chain management is required to prevent shortages of raw materials and reagents. Scaling up while preserving quality and avoiding contaminants (e.g., endotoxins) is technically demanding. Price pressures from procurers and the need for competitive costing in tender-driven markets create commercial challenges, and the field must continually demonstrate CRM197’s superiority versus alternative carriers to justify preference.
CRM197 Market: Segmentation Analysis
The global CRM197 market is segmented based on Type, Application, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2025 to 2034.
Based on Type, the global CRM197 market is divided into Research Grade CRM197, CGMP Grade CRM197.
On the basis of Application, the global CRM197 market is bifurcated into Meningococcal Polysaccharide Conjugate Vaccine, Pneumococcal Conjugate Vaccine.
The Regional, this segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America,and the Middle East and Africa.
CRM197 Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | CRM197 Market |
| Market Size in 2024 | USD 265.88 Million |
| Market Forecast in 2034 | USD 1800.57 Million |
| Growth Rate | CAGR of 21.08% |
| Number of Pages | 215 |
| Key Companies Covered | Xpress Biologics, Pfizer Inc., Fina Biosolutions LLC, Eubiologics, EirGenix Inc., Scarab Genomics, Beijing Kexing Biology Product, Ligand Pharmaceuticals, CanSino Biologic, and others. |
| Segments Covered | By Type, By Application, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
CRM197 Market: Regional Insights
The CRM197 market from 2025 to 2034 is expected to demonstrate strong regional growth patterns, with North America maintaining its dominance due to advanced biopharmaceutical infrastructure, significant R&D investments, and the presence of leading vaccine manufacturers. Europe is projected to show steady growth supported by stringent quality standards, established pharmaceutical hubs, and continuous advancements in vaccine conjugation technologies.
The Asia-Pacific region is anticipated to record the fastest growth rate owing to increasing vaccine production in countries such as China and India, growing healthcare awareness, and government immunization initiatives. Meanwhile, Latin America, the Middle East, and Africa are likely to experience moderate but promising growth driven by rising healthcare investments, expanding pharmaceutical industries, and efforts to enhance local vaccine manufacturing capabilities.
CRM197 Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the CRM197 market on a global and regional basis.
The global CRM197 market profiles key players such as:
- Xpress Biologics
- Pfizer Inc.
- Fina Biosolutions LLC
- Eubiologics
- EirGenix Inc.
- Scarab Genomics
- Beijing Kexing Biology Product
- Ligand Pharmaceuticals
- CanSino Biologic
The global CRM197 market is segmented as follows:
By Type
- Research Grade CRM197
- CGMP Grade CRM197
By Application
- Meningococcal Polysaccharide Conjugate Vaccine
- Pneumococcal Conjugate Vaccine
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
CRM197 is a non-toxic diphtheria toxin mutant that is used as a protein carrier for haptens and polysaccharides to make them immunogenic. Reportedly, the product is considered to be an ideal protein carrier and finds a slew of applications in approved conjugate vaccines that are used against encapsulated bacteria. Moreover, it is being assessed as a potential medicine-delivery fusion protein. In addition to this, CRM197 can also be utilized for preventing cancers having poor prognoses. It can be used in vaccines to make an individual immune to diseases such as pneumococcus, Haemophilus influenzae, and meningococcal meningitis.
The global CRM197 market is expected to grow due to rising demand for protein-based vaccines, increasing use in conjugate vaccine development, growth in immunization programs, and advancements in biopharmaceutical research.
According to a study, the global CRM197 market size was worth around USD 265.88 Million in 2024 and is expected to reach USD 1800.57 Million by 2034.
The global CRM197 market is expected to grow at a CAGR of 21.08% during the forecast period.
North America is expected to dominate the CRM197 market over the forecast period.
Leading players in the global CRM197 market include Xpress Biologics, Pfizer Inc., Fina Biosolutions LLC, Eubiologics, EirGenix Inc., Scarab Genomics, Beijing Kexing Biology Product, Ligand Pharmaceuticals, CanSino Biologic, among others.
The report explores crucial aspects of the CRM197 market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed