COVID-19 Saliva Screening Test Potential Market Size, Share, Trends, Growth 2032

COVID-19 Saliva Screening Test Potential Market By Technology (Fluorescence-labelled antigen, RT-PCR, and CRISPR-Cas9), By Mode of Testing (Centralized Testing and Decentralized Testing), And By Region- Global Industry Perspective, Comprehensive Analysis, and Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
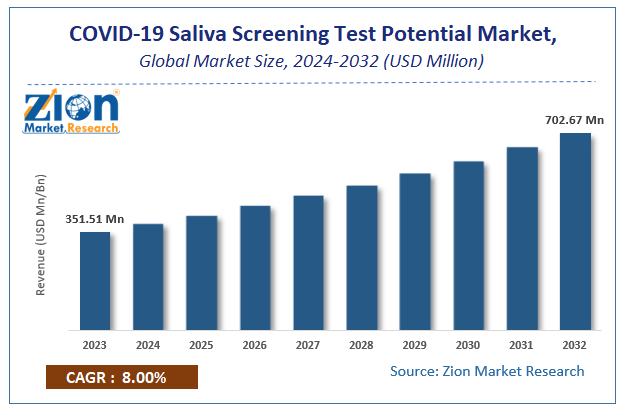
| USD 351.51 Million | USD 702.67 Million | 8% | 2023 |
COVID-19 Saliva Screening Test Potential Market Insights
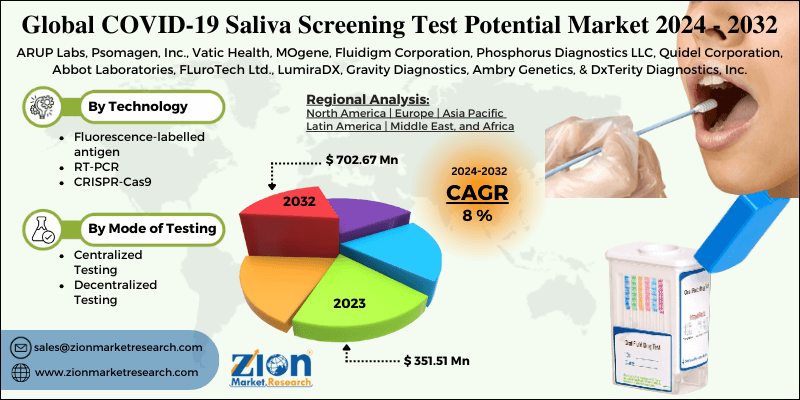
According to a report from Zion Market Research, the global COVID-19 Saliva Screening Test Potential Market was valued at USD 351.51 Million in 2023 and is projected to hit USD 702.67 Million by 2032, with a compound annual growth rate (CAGR) of 8% during the forecast period 2024-2032. This report explores market strengths, weakness, opportunities, and threats. It also provides valuable insights into the market's growth drivers, challenges, and the future prospects that may emerge in the COVID-19 Saliva Screening Test Potential Market industry over the next decade.
COVID-19 Saliva Screening Test Potential Market: Overview
According to WHO, SARS-CoV-2 is the key cause of COVID-19 and has spread across nearly 235 nations. Moreover, large-scale spread of the pandemic resulted in global lockdown in 2019-2020. Furthermore, WHO has recommended nasopharyngeal and oropharyngeal swab for quantitative assessment of SARS-CoV-2 RNA via RTPCR (real-time reverse transcription PCR). Reportedly, the virus is found to be constantly present in saliva & RTPCR of saliva specimens. Moreover, RTPCR of saliva specimens provides more benefits over oropharyngeal swab and nasopharyngeal swab such as saliva self-collection, cost-efficiency, and avoiding services of health workers for collecting specimen.
Additionally, salivary diagnosis includes “Salivaomics” that represents identifying of myriad “omics” constituting of salivary proteome, microRNA, microbiome, transcriptome, metabolome, and transcriptome. As per NCBI, saliva tests are found to be quick, cost-efficient, and highly sensitive & analytic in determination of oral ailments, viral infections, and human stress tests. Hence, saliva is considered to be effective diagnostic Biofluid for COVID-19 tests.
COVID-19 Saliva Screening Test Potential Market: Growth Dynamics
Accurate diagnosing of COVID 19 is key for containing disease in clinics and community set-up and testing of salivary specimens of individuals have proved to be technically advantageous as compared to testing of blood or any other body fluids. This, in turn, will drive growth of COVID-19 saliva screening test potential market over the years ahead. In addition to this, saliva can be easily self-collected by children & adults with patient spitting in sterile container.
Hence, collection of saliva eliminates requirement of services of healthcare professionals along with reducing nosocomial infections. This, in turn, will drive industry trends. Apparently, saliva screening minimizes costs and time related to specimen collection, thereby raising number of tests of patients along with supporting mass screening. This will create lucrative growth avenues for COVID-19 saliva screening test potential industry over the years ahead.
Furthermore, saliva specimen collection can be made when outpatient clinics, household areas, and community sites are not available. For the record, from saliva screening test results demonstrated by Australian researchers in June 2021 at Royal Melbourne Hospital, it was found that new saliva tests using infrared light technology had ability to detect COVID-19 within five minutes.
Such breakthroughs is likely to help the market explore new growth frontlines in the near future. Salivary specimen collection is non-invasive and can minimize exposure of health workers to COVID-19 disease to a large extent, thereby steering growth of industry sphere. The saliva collection can also be used for sequential determination of viral load and this will scale up market value in the next couple of years.
COVID-19 Saliva Screening Test Potential Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | COVID-19 Saliva Screening Test Potential Market |
| Market Size in 2023 | USD 351.51 Million |
| Market Forecast in 2032 | USD 702.67 Million |
| Growth Rate | CAGR of 8% |
| Number of Pages | 110 |
| Key Companies Covered | ARUP Labs, Psomagen, Inc., Vatic Health, MOgene, Fluidigm Corporation, Phosphorus Diagnostics LLC, Quidel Corporation, Abbot Laboratories, FLuroTech Ltd., LumiraDX, Gravity Diagnostics, Ambry Genetics, and DxTerity Diagnostics, Inc. |
| Segments Covered | By Technology, By Mode of Testing and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
COVID-19 Saliva Screening Test Potential Market: Regional Landscape
North American To Retain Market Domination Over 2024-2032
The growth of COVID-19 saliva screening test potential industry in North America during forecast period can be credited to launching of myriad products by startups. Apart from this, presence of key participants in the sub-continent involved in development of diagnostics & screening tests is likely to contribute majorly towards regional market size over the upcoming years.
COVID-19 Saliva Screening Test Potential Market: Competitive Space
The global COVID-19 Saliva Screening Test Potential market profiles key players such as:
- ARUP Labs
- Psomagen, Inc
- Vatic Health
- MOgene
- Fluidigm Corporation
- Phosphorus Diagnostics LLC
- Quidel Corporation
- Abbot Laboratories
- FLuroTech Ltd
- LumiraDX
- Gravity Diagnostics
- Ambry Genetics
- DxTerity Diagnostics, Inc
The global COVID-19 Saliva Screening Test Potential Market is segmented as follows:
By Technology
- Fluorescence-labelled antigen
- RT-PCR
- CRISPR-Cas9
By Mode of Testing
- Centralized Testing
- Decentralized Testing
By Region
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
What will be the CAGR value of the COVID-19 Saliva Screening Test Potential market during 2024-2032?
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed