Advanced Therapy Medicinal Products Market Size, Share, Trends, Growth and Forecast 2032

Advanced Therapy Medicinal Products Market By therapy type (somatic cell treatment product, quality treatment products, joined ATMPs and tissue engineered products) And By Region: - Global And Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, And Forecasts, 2024-2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 10.49 Billion | USD 24.93 Billion | 10.1% | 2023 |
Description
Advanced Therapy Medicinal Products Market Insights
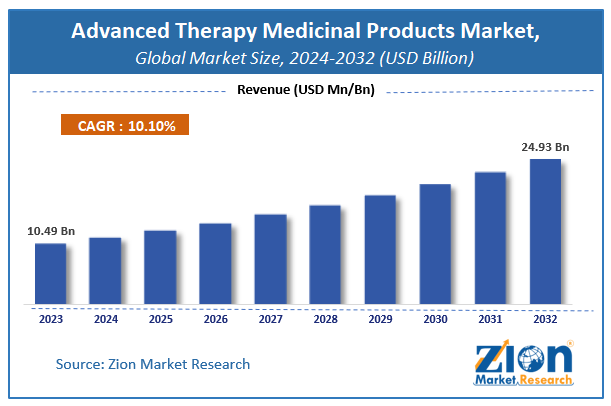
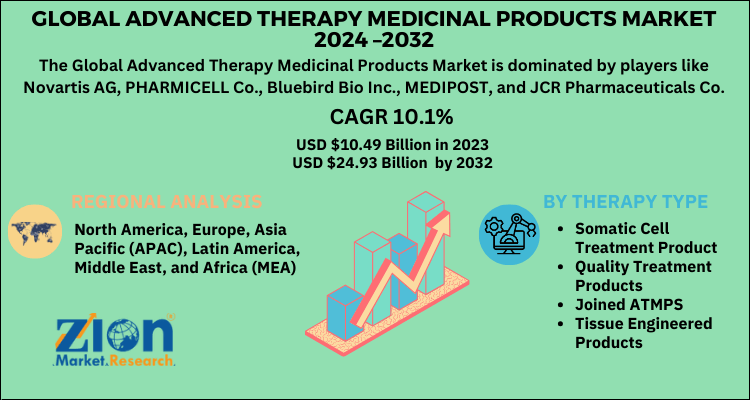
According to the report published by Zion Market Research, the global Advanced Therapy Medicinal Products Market size was valued at USD 10.49 Billion in 2023 and is predicted to reach USD 24.93 Billion by the end of 2032. The market is expected to grow with a CAGR of 10.1% during the forecast period. The report analyzes the global Advanced Therapy Medicinal Products Market's growth drivers, restraints, and impact on demand during the forecast period. It will also help navigate and explore the arising opportunities in the Advanced Therapy Medicinal Products industry.
Global Advanced Therapy Medicinal Products Market: Overview
The recent evolutions in immunotherapy have resulted in a slow transpose toward customized medicine from the traditional ‘one-size-fits-all’ approach. The advanced therapy medicinal product or ATMP perspective is one of the operative spaces in this newfound direction. These commodities offer solutions for disorders with negligible therapeutic substitutes as well, which is one of the prime stimulants for global advanced therapy medicinal products market.
Advanced therapy medicinal products are complete medications that depend on quality prescription, substantial cell treatment, and tissue-formulated items. This in turn will propel the growth of global advanced therapy medicinal products market in the forthcoming years.
Key Insights
- As per the analysis shared by our research analyst, the advanced therapy medicinal products market is anticipated to grow at a CAGR of 10.1% during the forecast period.
- The global advanced therapy medicinal products market was estimated to be worth approximately USD 10.49 billion in 2023 and is projected to reach a value of USD 24.93 billion by 2032.
- The growth of the advanced therapy medicinal products market is being driven by rising collaborations between biotech firms, pharmaceutical companies, and academic institutions.
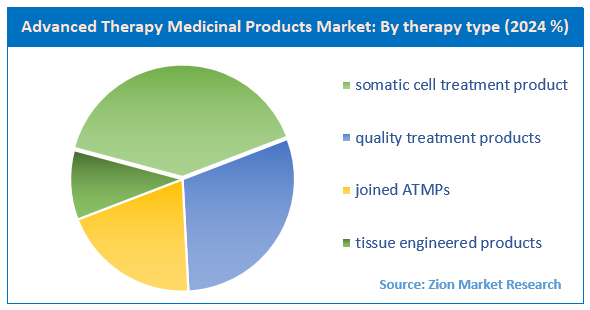
- Based on the therapy type, the somatic cell treatment product segment is growing at a high rate and is projected to dominate the market.
- By region, North America is expected to dominate the global market during the forecast period.
Global Advanced Therapy Medicinal Products Market: Growth Factors
Soaring costs of existing products and technological development in genetic tools have positively influenced the entry of new market players which in turn will lead to a remarkable growth of global advanced therapy medicinal products market in the upcoming years.
Of late, a notable stint has occurred in the global therapeutics market. People are turning toward customized treatments instead of pharmaceutical medications. This is because of the development in immunotherapy. Consequently, advanced therapy medicinal products market (ATMP) is booming, such commodities provide a fix for disorders with no medical substitutes. The already stated is a major element steering the expansion of the global advanced therapy medicinal products market universally.
The utilization of advanced therapy medicinal products (ATMPs) is proven to improve the quality of life and health status in future for patients suffering from life-threatening and chronic conditions. This is specifically for disorders with very little or no substitute treatment options. These innovative treatment modes deliver transformative superiority not offered by the traditional forms of disease treatment, thereby steering the ATMP market.
Additionally, lack of production capacities to meet the rising customer demand paired with the resource and budget restrictions faced by advanced therapy medicinal product manufacturers can hamper the progression of the market. Another restraint can be the fact that, in the current schema, ATMPs are costly for patient as well as for health insurance plans. However, surging competition to generate revenue share can help reduce costs and help the affordability, along with the embracement of these products as conventional medicine.
Advanced Therapy Medicinal Products Market Dynamics
Key growth drivers
The most significant driver for the ATMP market is the growing demand for personalized and innovative treatments for chronic and rare diseases. These therapies address the root cause of illnesses like genetic disorders and certain cancers, where traditional drugs have been ineffective. There's also increasing regulatory support from agencies like the FDA and EMA, which are creating accelerated approval pathways and providing incentives for the development of these therapies. This streamlined process encourages investment and speeds up market access. Finally, substantial advancements in biotechnology, such as CRISPR-Cas9 for gene editing and automated manufacturing processes, are enhancing the precision, efficacy, and scalability of ATMP production, further fueling market expansion.
Restraints
The market is significantly restrained by the exorbitantly high cost of development and manufacturing. The complex, patient-specific nature of many ATMPs requires specialized facilities and highly skilled personnel, leading to immense upfront costs that can make the therapies unaffordable for many healthcare systems and patients. This also creates a major hurdle for market access and reimbursement. Additionally, the stringent and complex regulatory and manufacturing requirements can be a major barrier. The unique biological nature of these products necessitates meticulous safety and quality controls, which can lead to lengthy and unpredictable approval timelines and complicate the scaling of production.
Opportunities
A key opportunity for the ATMP market is the expansion of manufacturing capabilities and the adoption of "off-the-shelf" allogeneic therapies. The development of closed and automated systems is helping to reduce the risk of contamination and improve scalability, making it possible to produce these therapies more efficiently. Allogeneic therapies, which use donor cells rather than patient-specific cells, can be mass-produced and are expected to significantly reduce costs and logistical challenges. Furthermore, the increasing number of clinical trials and research collaborations, particularly in emerging economies with large patient populations, creates new avenues for therapy development and market penetration.
Challenges
The ATMP market faces several critical challenges, including pricing and reimbursement complexities. The high, one-time treatment cost of many ATMPs doesn't fit neatly into traditional "pay-per-pill" healthcare models, making it difficult for payers to establish clear reimbursement policies. This uncertainty can limit patient access even after a therapy is approved. Another challenge is the difficulty in demonstrating long-term efficacy and safety. Since many of these therapies are curative or have long-lasting effects, gathering long-term clinical data to satisfy regulatory bodies and payers is a significant and time-consuming undertaking. This also presents ethical challenges for trial design, particularly in rare or life-threatening diseases with no standard of care.
Global Advanced Therapy Medicinal Products Market: Segmentation
On the basis of Therapy Type, the advanced therapy medicinal products market is categorized into somatic cell treatment product, quality treatment products, joined ATMPs and tissue engineered products.
Recent Development
- In April 2025, Artis BioSolutions officially entered the cell and gene therapy CDMO space through its acquisition of Landmark Bio. The deal brings capabilities in viral vectors, mRNA, lipid nanoparticles, and fill-finish operations, enabling Artis to offer end-to-end ATMP manufacturing solutions.
- In March 2025, AstraZeneca announced its agreement to acquire Belgian biotech EsoBiotec for up to USD 1 billion, aiming to accelerate its in vivo cell therapy programs, with a particular focus on oncology and autoimmune disorders.
- In late 2024, Roche reached a deal to acquire Poseida Therapeutics (U.S.-based) for up to USD 1.5 billion, expanding its pipeline in immune cell therapies, especially allogeneic CAR T platforms targeting hematologic malignancies and autoimmune diseases.
- In 2025, financially distressed Bluebird Bio agreed to be acquired by private equity firms Carlyle Group and SK Capital in a transaction valued at up to USD 96 million (including contingent payments). The acquisition aims to sustain Bluebird’s gene therapy portfolio, which includes programs for sickle cell disease, beta thalassemia, and related conditions.
- In 2023, The QbD Group acquired Michor Consulting, reinforcing its regulatory affairs capabilities for ATMPs across Europe. That same year, PharmaLex Group merged with Italy-based MAP Group to enhance market access services for innovative therapies, including ATMPs.
Advanced Therapy Medicinal Products Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Advanced Therapy Medicinal Products Market |
| Market Size in 2023 | USD 10.49 Billion |
| Market Forecast in 2032 | USD 24.93 Billion |
| Growth Rate | CAGR of 10.1% |
| Number of Pages | 206 |
| Key Companies Covered | Novartis AG, PHARMICELL Co., Bluebird Bio Inc., MEDIPOST, and JCR Pharmaceuticals Co. |
| Segments Covered | By therapy type and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Global Advanced Therapy Medicinal Products Market: Regional Analysis
Based on geographical analysis, the global advanced therapy medicinal products market is dominated by North America because of the proximity of well-known players and the development in caliber & cell treatment with medical help. Contrastingly, cell and quality treatment related constructive work in the space keeps on flourishing at a good pace, with numerous products progressing in clinical development.
Europe holds the second far-reaching pharmaceutical market volume globally. In the forthcoming period, cell therapy manufacturers and developers are predicted to hold a massive share in this region’s drug revenue. In addition, numerous academic institutions are conducting in-depth testing in first-stage cell therapy. The aforementioned constituent will fuel the growth of global advanced therapy medicinal products market in this region. Meanwhile, some players in global advanced therapy medicinal products market are aiming to enhance their command across Europe which will further assist market expansion.
Nonetheless, open doors are emerging in Asia Pacific countries. For instance, in nations like China, Japan, and India, the demand for advanced therapy medicinal products market is singularly high in these heavily populated nations. This is accredited to swift climb in medical services structure and rising levels of various sicknesses. In the African nations, the development of human services and the economic boom is driving the global advanced therapy medicinal products market.
Global Advanced Therapy Medicinal Products Market: Competitive Players
In the present times, the market is developing at a fast rate due to the strategic collaborations, acquisitions, and product launches undertaken by the prominent companies manufacturing these products. Some of the prominent players controlling the global advanced therapy medicinal products market include:
- Novartis AG
- PHARMICELL Co.
- Bluebird Bio Inc.
- MEDIPOST
- JCR Pharmaceuticals Co.
The Global Advanced Therapy Medicinal Products Market is segmented as follows:
By Therapy Type
- Somatic Cell Treatment Product
- Quality Treatment Products
- Joined ATMPs
- Tissue Engineered Products
By Region
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
What Reports Provides
- Full in-depth analysis of the parent market
- Important changes in market dynamics
- Segmentation details of the market
- Former, on-going, and projected market analysis in terms of volume and value
- Assessment of niche industry developments
- Market share analysis
- Key strategies of major players
- Emerging segments and regional markets
- Testimonials to companies in order to fortify their foothold in the market.
Table Of Content
FrequentlyAsked Questions
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed