Regulatory Affairs Outsourcing Market Size, Share | 2032

Regulatory Affairs Outsourcing Market By Services (Regulatory Consulting, Legal Representation, Regulatory Writing & Publishing, Product Registration & Clinical Trial Application, and Other Regulatory Services), and By Region: Global Industry Perspective, Comprehensive Analysis and Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 10.18 Billion | USD 21.21 Billion | 8.5% | 2023 |
Regulatory Affairs Outsourcing Market: Industry Perspective
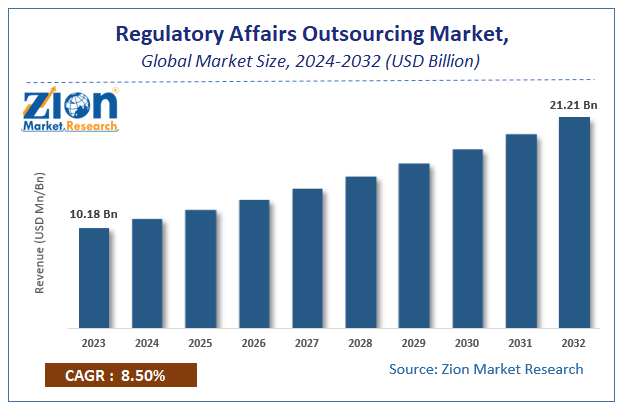
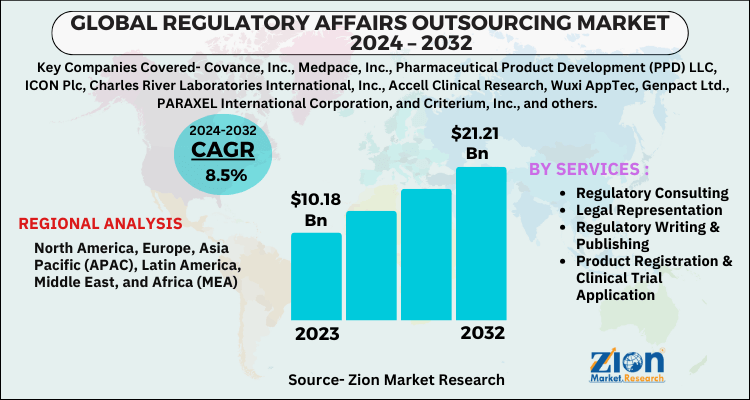
The global regulatory affairs outsourcing market size was worth around USD 10.18 Billion in 2023 and is predicted to grow to around USD 21.21 Billion by 2032 with a compound annual growth rate (CAGR) of roughly 8.5% between 2024 and 2032. The report analyzes the global regulatory affairs outsourcing market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the regulatory affairs outsourcing industry.
The report covers forecasts and analyses for the global regulatory affairs outsourcing market. The study provides historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on revenue (USD Billion). In-depth secondary research is used to ascertain the overall market size, top industry players, top products, industry associations, etc. Macroeconomic indicators such as healthcare industry outlook, healthcare spending, research funding, and GDP along with company websites, company annual reports, white papers, financial reports and other sources have also been considered to arrive at the indicated market numbers.
Along with pharmacovigilance and regulatory affairs, outsourcing is gradually becoming an exceptionally prevalent practice in the biotech and pharmaceutical industries. Regulatory outsourcing has become a norm as several businesses are seeking partners to handle any kind of operational duties that include submitting, publishing and publishing reports. Small and large life science firms prefer to outsource suppliers to handle tactical duties while helping to implement best practices.
Regulatory Affairs Outsourcing Market: Segmentation Analysis
The study provides a decisive view of the regulatory affairs outsourcing market by segmenting the market based on services, and regions. All the segments have been analyzed based on present and future trends and the market is estimated from 2024 to 2032. Based on services the market is segmented into regulatory consulting, legal representation, product registration & clinical trial application, regulatory writing & publishing, and other regulatory services. Regulatory writing & publication retained the highest market share in 2023 and over the forecast period is anticipated to continue to lead.
The segment is expected to account for a market share of more than 39.2% by 2032. Legal representation over the forecast period is expected to be the fastest-increasing segment.
Regulatory Affairs Outsourcing Market: Regional Analysis
Increasing demand for legal representatives in advanced regions such as Europe and North America is getting traction for market permission ideas for businesses that want to set up a base in the nation concerned. Regional segmentation includes the current and forecast demand for Asia Pacific, North America, Latin America, Europe, and Middle East & Africa with its further bifurcation into major countries.
The study also includes drivers and restraints for regulatory affairs outsourcing along with the impact they have on the demand over the forecast period. Besides, the report includes the study of opportunities and trends available in the regulatory affairs outsourcing market on a global level. The regulatory affairs outsourcing industry, especially in clinical research organizations, is anticipated to experience important growth owing to services.
Regulatory Affairs Outsourcing Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Regulatory Affairs Outsourcing Market |
| Market Size in 2023 | USD 10.18 Billion |
| Market Forecast in 2032 | USD 21.21 Billion |
| Growth Rate | CAGR of 8.5% |
| Number of Pages | 210 |
| Key Companies Covered | Covance, Inc., Medpace, Inc., Pharmaceutical Product Development (PPD) LLC, ICON Plc, Charles River Laboratories International, Inc., Accell Clinical Research, Wuxi AppTec, Genpact Ltd., PARAXEL International Corporation, and Criterium, Inc., and others |
| Segments Covered | By Services and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
The increasing numbers of patent expirations with increasing R&D spending are the most significant variables that influence the growth of the outsourcing industry for worldwide regulatory relations. Biopharmaceutical and healthcare organizations are planning to collaborate with several outsourcing firms to approve their equipment and drugs on the worldwide market.
A device or drug's authorization time is considered time-consuming, and expensive, as well as a documentation-centered method. Expert service providers are increasingly required to specialize primarily in information processing associated with regulatory affairs, thereby further improving the need for reliable service suppliers to outsource regulatory affairs.
Regulatory Affairs Outsourcing Market: Competitive Analysis
The report also provides a company market share analysis to give a broader view of the key players in the market. Industry insights and information are delivered in the required format. ZMR develops a list of industry players (manufacturers), distributors, retailers and industry experts.
Some of the major players in the global regulatory affairs outsourcing market include:
- Covance Inc.
- Medpace Inc.
- Pharmaceutical Product Development (PPD) LLC
- ICON Plc
- Charles River Laboratories International Inc.
- Accell Clinical Research
- Wuxi AppTec
- Genpact Ltd.
- PARAXEL International Corporation
- Criterium Inc.
This report segments the global regulatory affairs outsourcing market as follows:
By Services Segment Analysis
- Regulatory Consulting
- Legal Representation
- Regulatory Writing & Publishing
- Product Registration & Clinical Trial Application
- Other Regulatory Services
By Regional Segment Analysis
- North America
- U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- Middle East and Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Regulatory affairs outsourcing is the practice of hiring an external company or service provider to handle specific or all aspects of the regulatory processes involved in bringing a product or service to market. This is particularly common in industries with complex and ever-changing regulatory landscapes, such as pharmaceuticals, medical devices, and food and beverage.
According to a study, the global regulatory affairs outsourcing market size was worth around USD 10.18 billion in 2023 and is expected to reach USD 21.21 billion by 2032.
The global regulatory affairs outsourcing market is expected to grow at a CAGR of 8.5% during the forecast period.
Europe and North America are expected to dominate the regulatory affairs outsourcing market over the forecast period.
Leading players in the global regulatory affairs outsourcing market include Covance, Inc., Medpace, Inc., Pharmaceutical Product Development (PPD) LLC, ICON Plc, Charles River Laboratories International, Inc., Accell Clinical Research, Wuxi AppTec, Genpact Ltd., PARAXEL International Corporation, and Criterium, Inc., among others.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed