Global Newborn Screening Instruments Market Size, Share, Growth Analysis Report - Forecast 2034

Newborn Screening Instruments Market By Product (Instruments, Reagents), By Technology (Tandem Mass Spectrometry, Pulse Oximetry, Enzyme Based Assay, DNA Assay, Electrophoresis, Others), By Test Type (Dry Blood Spot Test, CCHD, Hearing Screen), and By Region: Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2025 - 2034
| Market Size in 2024 | Market Forecast in 2034 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 1029.6 Million | USD 2543.14 Million | 8.7% | 2024 |
Global Newborn Screening Instruments Market: Industry Perspective
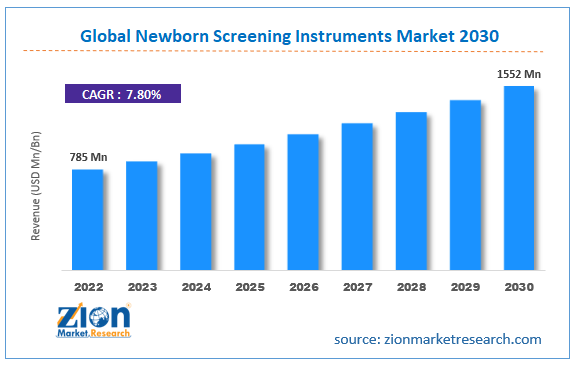
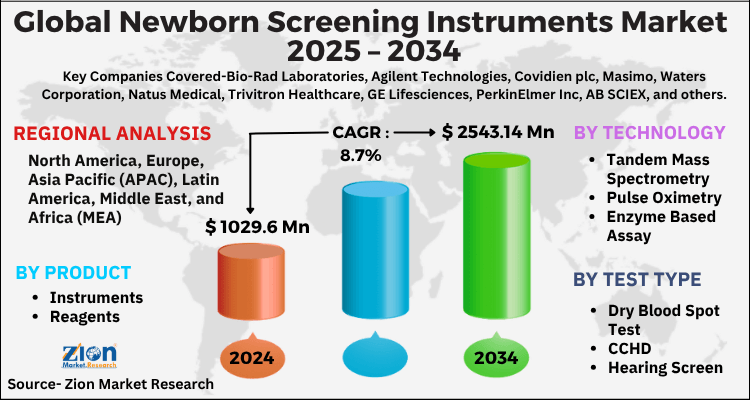
The global newborn screening instruments market size was worth around USD 1029.6 Million in 2024 and is predicted to grow to around USD 2543.14 Million by 2034 with a compound annual growth rate (CAGR) of roughly 8.7% between 2025 and 2034. The report analyzes the global newborn screening instruments market's drivers, restraints/challenges, and the effect they have on the demands during the projection period. In addition, the report explores emerging opportunities in the newborn screening instruments industry.
Global Newborn Screening Instruments Market: Overview
Newborn screening instruments are medical devices designed to identify certain genetic, metabolic, and congenital disorders in newborn babies shortly after birth. This early detection enables timely intervention and treatment, potentially preventing serious health complications or even death. These instruments are an integral part of newborn screening programs implemented in many countries to ensure the health and well-being of newborns.
Newborn screening instruments typically involve the collection of a small blood sample from the baby's heel. This sample is then analyzed using various techniques, including mass spectrometry, tandem mass spectrometry (MS/MS), fluorescence, and enzymatic assays. The instruments can detect a range of conditions such as phenylketonuria (PKU), congenital hypothyroidism, sickle cell disease, and metabolic disorders. The process starts with the collection of a few drops of blood on filter paper, which is then dried and sent to a laboratory. The laboratory technicians use specialized equipment, often referred to as newborn screening instruments, to analyze the dried blood spots. These instruments can rapidly and accurately measure the levels of various substances or markers in the blood that indicate the presence of specific disorders.
Global Newborn Screening Instruments Market: Growth Drivers
Rising incidence of neonatal disorders to drive market growth during the forecast period.
The rising incidence of neonatal disorders globally has emerged as a significant driver propelling the growth of the newborn screening instrument market. Neonatal disorders encompass a range of congenital, genetic, and metabolic conditions that can have long-term detrimental effects on an infant's health and development if not identified and treated early. With increasing awareness about the importance of early detection and intervention, there has been a surge in demand for efficient and comprehensive newborn screening procedures. Statistics pertaining to the prevalence of neonatal disorders reveal a concerning trend. According to global health organizations and studies, the prevalence of congenital disorders and metabolic conditions in newborns varies by region but is estimated to be around 2-5% worldwide. Moreover, the occurrence of these disorders is on the rise due to factors such as changing lifestyles, consanguineous marriages, and genetic predisposition. This escalation in neonatal disorders underscores the urgent need for robust newborn screening programs that can accurately identify these conditions shortly after birth, enabling healthcare professionals to initiate timely interventions that can significantly improve outcomes.
Restraints:
Cost and infrastructure challenges may hamper the market expansion
Implementing newborn screening programs requires substantial investment in purchasing screening instruments, training healthcare professionals, and establishing the necessary infrastructure. The average cost of newborn screening instruments varies depending on the type of instrument and the number of disorders that it can screen for. However, it is generally in the range of USD 5,000 to USD 10,000 per instrument. The cost of infrastructure for a newborn is typically in the range of USD 100,000 to USD 500,000. The cost of acquiring and maintaining advanced screening technologies can be a deterrent for healthcare facilities, especially in resource-limited settings. Beyond the initial investment, there are ongoing operational costs associated with maintaining and servicing screening instruments, conducting tests, and interpreting results. These costs can strain healthcare budgets, particularly in regions with limited financial resources. Healthcare facilities in certain regions, especially in rural and remote areas, may lack the necessary infrastructure and trained personnel to conduct effective newborn screening. The absence of adequate laboratory facilities, skilled technicians, and transportation logistics can hinder the implementation of screening programs.
Opportunities:
Advancements in genetic research to provide several growth opportunities
As the understanding of genetics and genomics continues to expand, new insights into the genetic basis of neonatal disorders are emerging. New sequencing technologies are making it possible to sequence the genomes of newborns at an affordable cost. This allows researchers to identify genetic mutations that are associated with neonatal disorders. For example, in 2023, Illumina announced the launch of its NovaSeq 6000 system, which can sequence the genomes of newborns in a few hours. This presents an opportunity to develop more targeted and comprehensive newborn screening tests. By incorporating the latest genetic findings, screening instruments can be designed to identify a broader range of disorders with higher accuracy and sensitivity. Moreover, these advancements enable the identification of specific genetic markers associated with various conditions, allowing for early detection even before clinical symptoms manifest. This not only aids in timely intervention but also lays the foundation for personalized medicine approaches tailored to an individual's genetic makeup. As research breakthroughs continue to unveil the complex genetic underpinnings of neonatal disorders, the newborn screening instrument market can harness this knowledge to offer more sophisticated and effective screening solutions, ultimately improving healthcare outcomes for newborns around the world.
Challenges:
Standardization of protocols to challenge market cap growth
Standardization of protocols poses a significant challenge for the newborn screening instrument market, impacting the consistency and reliability of screening programs. The lack of uniform guidelines and protocols for screening tests across different regions and healthcare settings can lead to variations in testing procedures, result interpretation, and follow-up actions. This variability hampers the ability to compare results accurately, making it difficult to establish a standardized approach to newborn screening. As a consequence, the quality and accuracy of screening outcomes can be compromised, leading to discrepancies in the identification and diagnosis of neonatal disorders. Moreover, the absence of standardized protocols can create confusion among healthcare professionals and caregivers, potentially resulting in missed diagnoses or unnecessary interventions. It also complicates efforts to collect and analyze data on a broader scale for research and public health purposes. Addressing the challenge of standardization requires collaborative efforts among healthcare authorities, medical societies, and industry stakeholders to develop and promote consistent guidelines and protocols. Only through standardized procedures can the newborn screening instrument market ensure the highest level of accuracy, reliability, and comparability of screening results, thus improving the effectiveness of newborn screening programs and the well-being of newborns worldwide.
Key Insights
- As per the analysis shared by our research analyst, the global newborn screening instruments market is estimated to grow annually at a CAGR of around 8.7% over the forecast period (2025-2034).
- Regarding revenue, the global newborn screening instruments market size was valued at around USD 1029.6 Million in 2024 and is projected to reach USD 2543.14 Million by 2034.
- The newborn screening instruments market is projected to grow at a significant rate due to rising prevalence of genetic disorders, government initiatives for early disease detection, technological advancements, and increasing birth rates in emerging economies.
- Based on Product, the Instruments segment is expected to lead the global market.
- On the basis of Technology, the Tandem Mass Spectrometry segment is growing at a high rate and will continue to dominate the global market.
- Based on the Test Type, the Dry Blood Spot Test segment is projected to swipe the largest market share.
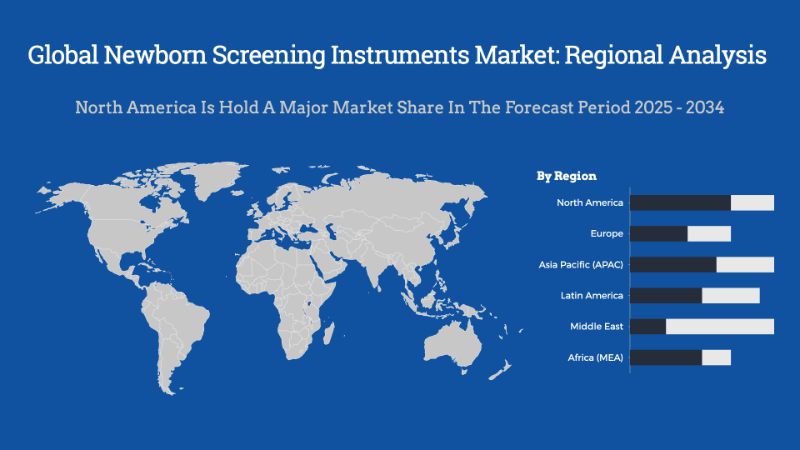
- Based on region, North America is predicted to dominate the global market during the forecast period.
Global Newborn Screening Instruments Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Newborn Screening Instruments Market |
| Market Size in 2024 | USD 1029.6 Million |
| Market Forecast in 2034 | USD 2543.14 Million |
| Growth Rate | CAGR of 8.7% |
| Number of Pages | 212 |
| Key Companies Covered | Bio-Rad Laboratories, Agilent Technologies, Covidien plc, Masimo, Waters Corporation, Natus Medical, Trivitron Healthcare, GE Lifesciences, PerkinElmer Inc, AB SCIEX, and others. |
| Segments Covered | By Product, By Technology, By Test Type, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2024 |
| Historical Year | 2020 to 2023 |
| Forecast Year | 2025 - 2034 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Newborn Screening Instruments Market: Segmentation Analysis
The global newborn screening instruments market is segmented based on Product, Technology, Test Type, and region. All the segments have been analyzed based on present and future trends and the market is estimated from 2025 to 2034.
Based on Product, the global newborn screening instruments market is divided into Instruments, Reagents.
On the basis of Technology, the global newborn screening instruments market is bifurcated into Tandem Mass Spectrometry, Pulse Oximetry, Enzyme Based Assay, DNA Assay, Electrophoresis, Others.
By Test Type, the global newborn screening instruments market is split into Dry Blood Spot Test, CCHD, Hearing Screen.
The Regional, this segment includes the current and forecast demand for North America, Europe, Asia Pacific, Latin America,and the Middle East and Africa.
Global Newborn Screening Instruments Market: Regional Analysis
The newborn screening instruments market shows varied growth across regions, driven by differences in healthcare infrastructure, government initiatives, and awareness levels. North America holds a significant share due to well-established healthcare systems, mandatory screening programs, and strong technological adoption. Europe follows closely, supported by government policies promoting early detection of metabolic and genetic disorders. Meanwhile, Asia-Pacific is witnessing rapid growth fueled by increasing birth rates, expanding healthcare access, and rising awareness about early diagnosis, though disparities in screening coverage persist across countries. Latin America, the Middle East, and Africa are developing markets where progress is slower but gaining momentum as governments prioritize neonatal health and invest in modern screening technologies. Overall, global demand for newborn screening instruments is expected to rise steadily, propelled by a growing emphasis on early disease detection and improving child health outcomes worldwide.
Global Newborn Screening Instruments Market: Recent Developments
- In August 2024, Trivitron Healthcare launched Centre of Excellence (CoE) in genomics, newborn screening, metabolomics, and molecular diagnostics. This CoE will focus on creating unparalleled medical discoveries to transform healthcare delivery.
Global Newborn Screening Instruments Market: Competitive Analysis
The report provides a company market share analysis to give a broader overview of the key market players. In addition, the report also covers key strategic developments of the market, including acquisitions & mergers, new product launches, agreements, partnerships, collaborations & joint ventures, research & development, and regional expansion of major participants involved in the newborn screening instruments market on a global and regional basis.
The global newborn screening instruments market is dominated by players like:
- Bio-Rad Laboratories
- Agilent Technologies
- Covidien plc
- Masimo
- Waters Corporation
- Natus Medical
- Trivitron Healthcare
- GE Lifesciences
- PerkinElmer Inc
- AB SCIEX
Global Newborn Screening Instruments Market: Segmentation Analysis
The global newborn screening instruments market is segmented as follows;
By Product
- Instruments
- Reagents
By Technology
- Tandem Mass Spectrometry
- Pulse Oximetry
- Enzyme Based Assay
- DNA Assay
- Electrophoresis
- Others
By Test Type
- Dry Blood Spot Test
- CCHD
- Hearing Screen
Global Newborn Screening Instruments Market: Regional Segment Analysis
- North America
- The U.S.
- Canada
- Mexico
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- Australia
- South Korea
- Rest of Asia Pacific
- The Middle East & Africa
- Saudi Arabia
- UAE
- Egypt
- Kuwait
- South Africa
- Rest of the Middle East & Africa
- Latin America
- Brazil
- Argentina
- Rest of Latin America
Table Of Content
Methodology
FrequentlyAsked Questions
Newborn screening instruments are medical devices designed to identify certain genetic, metabolic, and congenital disorders in newborn babies shortly after birth. This early detection enables timely intervention and treatment, potentially preventing serious health complications or even death. These instruments are an integral part of newborn screening programs implemented in many countries to ensure the health and well-being of newborns.
The global newborn screening instruments market is expected to grow due to increasing government initiatives supporting early disease detection, rising incidence of neonatal disorders, advancements in screening technologies.
According to a study, the global newborn screening instruments market size was worth around USD 1029.6 Million in 2024 and is expected to reach USD 2543.14 Million by 2034.
The global newborn screening instruments market is expected to grow at a CAGR of 8.7% during the forecast period.
North America is expected to dominate the newborn screening instruments market over the forecast period.
Leading players in the global newborn screening instruments market include Bio-Rad Laboratories, Agilent Technologies, Covidien plc, Masimo, Waters Corporation, Natus Medical, Trivitron Healthcare, GE Lifesciences, PerkinElmer Inc, AB SCIEX, among others.
The report explores crucial aspects of the newborn screening instruments market, including a detailed discussion of existing growth factors and restraints, while also examining future growth opportunities and challenges that impact the market.
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed