Hydroxychloroquine Market Size, Share & Growth Report 2032

Hydroxychloroquine Market By Type (USP Standards Grade, Pharmaceutical Standards Grade, EP Standards Grade, And Others), By Application (Rheumatoid Arthritis, Skin Lesions, Systemic Lupus Erythematosus, Chronic Discoid Lupus Erythematosus, And Others), And By Region - Global Industry Perspective, Comprehensive Analysis, And Forecast, 2024 - 2032
| Market Size in 2023 | Market Forecast in 2032 | CAGR (in %) | Base Year |
|---|---|---|---|
| USD 941.1 Million | USD 1,405.60 Million | 4.68% | 2023 |
Hydroxychloroquine Market: Industry Perspective
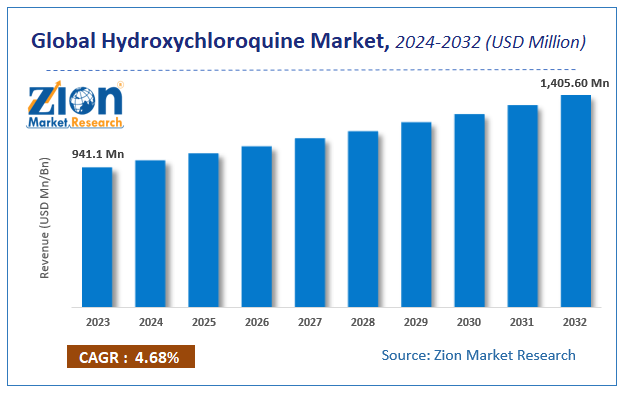
The global hydroxychloroquine market size was worth around USD 941.1 million in 2023 and is predicted to grow to around USD 1,405.60 million by 2032 with a compound annual growth rate (CAGR) of roughly 4.68% between 2024 and 2032.
The report offers a valuation and analysis of the hydroxychloroquine market on a global as well as regional level. The study offers a comprehensive assessment of the industry competition, restraints, revenue estimates, avenues, current & emerging trends, and industry-validated market information. The report offers historical data from 2018 to 2022 along with a forecast from 2024 to 2032 based on value (USD Million).
Hydroxychloroquine Market: ?Introduction
Hydroxychloroquine also referred to as Hydroxychloroquine phosphate is a drug that is used for treating malaria and certain auto-immune disorders including lupus & rheumatoid arthritis. Moreover, the medicine is part of disease-modifying anti-rheumatic drugs. It can alleviate skin problems in lupus and avoid pain or swelling caused due to arthritis. However, the drug is not approved by the U.S. FDA to treat coronavirus.
Furthermore, Hydroxychloroquine is recently studied as the best possible drug for treating COVID-19. The generic form of the drug is also available and it can also be taken orally. The medicine can also be utilized as a part of combination therapy. Its medical application was approved in the U.S. in 1955 to treat many chronic diseases.
Hydroxychloroquine Market: Growth Dynamics
According to NIH, Hydroxychloroquine is used for treating malaria as well as rheumatoid conditions. Various studies conducted by NIH concluded that the medicine displayed anti-viral features and the capability to alter immune system activities along with establishing of safe profile at apt dosages. This, in turn, is likely to enhance the drug's potential and its effectiveness in treating novel coronavirus.
Moreover, Hydroxychloroquine is found to have fewer side effects and low toxicity even if overdosed. Developed nations have a good supply of drugs due to the huge presence of drug manufacturers in these nations. Reportedly, when the combination of Hydroxychloroquine and azithromycin drug treatment was given to nearly 40 COVID-19 patients in France, it helped in clearing the virus from their bodies and this was concluded through the nasal swabs samples of the patients. For the record, the U.S. has been using it as a preventive medicine against COVID-19 recently.
Hospitals in the U.S. are storing the drug after the U.S. president termed the drug as the game-changer in treating of coronavirus. Even some countries of Europe along with China and South Korea have recommended the use of the drug for treating COVID-19 patients. Moreover, the drug has been advocated in countries like India as a measure to prevent the coronavirus in people who have been in contact with COVID-19-positive persons and those falling in high-risk zones. All these aforementioned aspects are likely to favorably leverage the growth of the Hydroxychloroquine market over the forecast period.
Hydroxychloroquine Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Hydroxychloroquine Market |
| Market Size in 2023 | USD 941.1 Million |
| Market Forecast in 2032 | USD 1,405.60 Million |
| Growth Rate | CAGR of 4.68% |
| Number of Pages | 240 |
| Key Companies Covered | Teva, Mylan, Novartis, Cadila Healthcare, Torrent Pharma, Shanghai Zhongxisanwei, BSE healthcare, Sandoz, HIKMA, IPCA Laboratories, Cipla Limited, Shanghai Pharma, Intas Pharmaceuticals, Lupin Limited, Shenhua Pharm, Sanofi, H-QYN, Macleod Pharmaceuticals, McW Healthcare, Laurus Labs, TAJ Pharma, Wuhan Wuyao Pharmaceutical, MAAN Medex, Cinkate, Concordia Healthcare, and others. |
| Segments Covered | By Type, By Application, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, The Middle East and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Hydroxychloroquine Market: Regional Analysis
- Asia Pacific To Contribute Lucratively Towards Market Share By 2032
The growth of the Hydroxychloroquine market in the region can be attributed to India being the leading producer of the drug and China being the largest exporter of the raw materials for the drug production. Apart from this, it is the most cost-effective drug in India costing nearly less than rupees three per tablet.
According to Forbes, India produces 70% of the drug and banned it in March 2020. However, U.S. President Trump requested the supply of the drug from India as he considered it a game-changer in medicine history and in treating coronavirus. For the record, the U.S. government has placed an order for 29 million doses of the drug to be imported from India. This resulted in the lifting of the export ban by the country for India’s neighbors as well as the U.S. Apart from this, nearly 36 countries have demanded the drug and including Italy, Spain, Australia, Germany, Israel, Indonesia and the U.S. These abovementioned factors will drive the business growth in Asia Pacific over the forthcoming years.
As per the authentic sources, India is expected to export the drugs to more than 20 countries including Mauritius, U.S., Canada, Seychelles, Brazil, UK, Bangladesh, Spain, Afghanistan, Germany, France, Bahrain, Australia, Nepal, Bhutan, Maldives, Dominican Republic, Israel, New Zealand and South Africa for helping these countries combat novel coronavirus effectively. This aspect will create lucrative growth avenues for the market in the Asia Pacific over the ensuing years.
Furthermore, the presence of leading API manufacturers such as Mangalam Drugs, Laurus Labs, Abbott India, Rusan Pharma, Unichem Remedies, and Vijayasri Organics in India will contribute notably towards the market earnings in the Asia Pacific region. The country also has the established presence of key drug manufacturers like IPCA, Laurus Labs, and Zydus Cadila.
Hydroxychloroquine Market: Competitive Analysis
The global hydroxychloroquine market is dominated by players like:
- Teva
- Mylan
- Novartis
- Cadila Healthcare
- Torrent Pharma
- Shanghai Zhongxisanwei
- BSE healthcare
- Sandoz
- HIKMA
- IPCA Laboratories
- Cipla Limited
- Shanghai Pharma
- Intas Pharmaceuticals
- Lupin Limited
- Shenhua Pharm
- Sanofi
- H-QYN
- Macleod Pharmaceuticals
- McW Healthcare
- Laurus Labs
- TAJ Pharma
- Wuhan Wuyao Pharmaceutical
- MAAN Medex
- Cinkate
- Concordia Healthcare
The global Hydroxychloroquine market is segmented as follows:
By Type Segment Analysis
- USP Standards Grade
- Pharmaceutical Standards Grade
- EP Standards Grade
- Others
By Application Segment Analysis
- Rheumatoid Arthritis
- Skin Lesions
- Systemic Lupus Erythematosus
- Chronic Discoid Lupus Erythematosus
- Others
By Regional Segment Analysis
- North America
- The U.S.
- Canada
- Europe
- France
- The UK
- Spain
- Germany
- Italy
- Rest of Europe
- Asia Pacific
- China
- Japan
- India
- South Korea
- Southeast Asia
- Rest of Asia Pacific
- Latin America
- Brazil
- Mexico
- Rest of Latin America
- Middle East & Africa
- GCC
- South Africa
- Rest of Middle East & Africa
Table Of Content
Methodology
FrequentlyAsked Questions
Hydroxychloroquine is a medication that wears two hats in the medical world. Primarily, it's used to combat malaria. In areas where malaria is prevalent, hydroxychloroquine can be taken to prevent infection. It can also treat uncomplicated cases of malaria if the disease is already contracted.
According to a study, the global hydroxychloroquine market size was worth around USD 941.1 million in 2023 and is expected to reach USD 1,405.60 million by 2032.
The global hydroxychloroquine market is expected to grow at a CAGR of 4.68% during the forecast period.
Asia Pacific is expected to dominate the hydroxychloroquine market over the forecast period.
Leading players in the global hydroxychloroquine market include Teva, Mylan, Novartis, Cadila Healthcare, Torrent Pharma, Shanghai Zhongxisanwei, BSE Healthcare, Sandoz, HIKMA, IPCA Laboratories, Cipla Limited, Shanghai Pharma, Intas Pharmaceuticals, Lupin Limited, Shenhua Pharm, Sanofi, H-QYN, Macleod Pharmaceuticals, McW Healthcare, Laurus Labs, TAJ Pharma, Wuhan Wuyao Pharmaceutical, MAAN Medex, Cinkate, and Concordia Healthcare, among others.
The hydroxychloroquine market report covers the geographical market along with a comprehensive competitive landscape analysis. It also includes cash flow analysis, profit ratio analysis, market basket analysis, market attractiveness analysis, sentiment analysis, PESTLE analysis, trend analysis, SWOT analysis, trade area analysis, demand & supply analysis, Porter’s five forces analysis, and value chain analysis.
RelatedNews
HappyClients
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed