Hemophilia B Gene Therapy Market Is Predicted To Register A CAGR Of 23.60% From 2024 To 2032
25-Jun-2025 | Zion Market Research
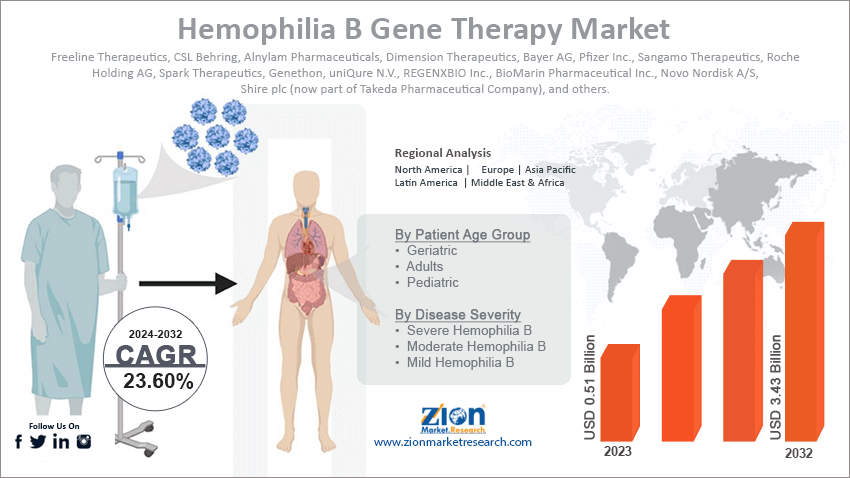
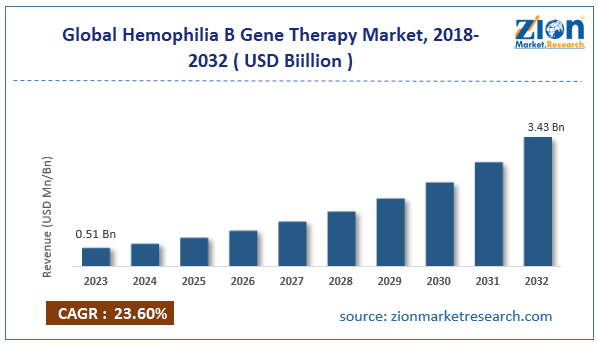
The global hemophilia B gene therapy market size was worth around USD 0.51 billion in 2023 and is predicted to grow to around USD 3.43 billion by 2032, with a compound annual growth rate (CAGR) of roughly 23.60% between 2024 and 2032.
Hemophilia B gene therapy represents treatment approaches for blood clotting disorders through genetic modification techniques that restore normal coagulation function. Recent developments include improvements in adeno-associated virus vector technology, enhanced gene delivery systems, and personalized treatment plans. Advanced manufacturing and better safety profiles have increased efficacy and reduced side effects. Gene therapy is now more accessible, effective, reliable, and transformative for patients with severe bleeding disorders.
Browse the full “Hemophilia B Gene Therapy Market By Patient Age Group (Geriatric, Adults, and Pediatric), By Disease Severity (Severe Hemophilia B, Moderate Hemophilia B, and Mild Hemophilia B), and By Region - Global and Regional Industry Overview, Market Intelligence, Comprehensive Analysis, Historical Data, and Forecasts 2024 - 2032”- Report at https://www.zionmarketresearch.com/report/hemophilia-b-gene-therapy-market
The growth of the global hemophilia B gene therapy industry is primarily driven by the increasing prevalence of inherited bleeding disorders, advancing genetic engineering technologies, and growing healthcare investment in precision medicine solutions.
Market Growth Factors
Several factors are propelling the expansion of the hemophilia B gene therapy market.
- Treatment effectiveness: Gene therapy offers long-term or even permanent relief, reducing the need for frequent factor replacement and greatly improving patients’ quality of life.
- Medical advancement: Ongoing research in genetic medicine is leading to more advanced and targeted treatments with better safety and effectiveness.
- Healthcare investment: Strong funding from both government and private investors is speeding up clinical trials, regulatory approval, and the commercial launch of new therapies.
Restraints
- Development costs: High expenses from research, testing, and meeting regulations create financial hurdles, limiting how companies can develop these treatments.
- Regulatory complexity: Strict approval rules and safety checks make development slow and create uncertainty about when new treatments can reach the market.
Hemophilia B Gene Therapy Market: Report Scope
| Report Attributes | Report Details |
|---|---|
| Report Name | Hemophilia B Gene Therapy Market |
| Market Size in 2023 | USD 0.51 Billion |
| Market Forecast in 2032 | USD 3.43 Billion |
| Growth Rate | CAGR of 23.60% |
| Number of Pages | 225 |
| Key Companies Covered | Freeline Therapeutics, CSL Behring, Alnylam Pharmaceuticals, Dimension Therapeutics, Bayer AG, Pfizer Inc., Sangamo Therapeutics, Roche Holding AG, Spark Therapeutics, Genethon, uniQure N.V., REGENXBIO Inc., BioMarin Pharmaceutical Inc., Novo Nordisk A/S, Shire plc (now part of Takeda Pharmaceutical Company), and others. |
| Segments Covered | By Patient Age Group, By Disease Severity, and By Region |
| Regions Covered | North America, Europe, Asia Pacific (APAC), Latin America, Middle East, and Africa (MEA) |
| Base Year | 2023 |
| Historical Year | 2018 to 2022 |
| Forecast Year | 2024 - 2032 |
| Customization Scope | Avail customized purchase options to meet your exact research needs. Request For Customization |
Market Segmentation
The hemophilia B gene therapy market can be segmented by therapy type, delivery method, distribution channel, patient population, and region.
Based on therapy type, the market is divided into adeno-associated virus therapies, lentiviral vector treatments, gene editing approaches, protein replacement therapies, and combination treatment protocols. Adeno-associated virus therapies are expected to lead the market during the forecast period due to their proven safety profiles and successful clinical trial outcomes.
Based on delivery method, the hemophilia B gene therapy industry is categorized into intravenous administration, intramuscular injection, hepatic delivery systems, targeted cell therapy, and novel delivery platforms. Intravenous administration leads the market due to its established clinical protocols and the familiarity of healthcare providers with administration procedures.
Based on distribution channels, the market is segmented into hospital pharmacies, specialty treatment centers, clinical research facilities, pharmaceutical distributors, and direct manufacturer programs. Hospital pharmacies lead the market, as gene therapies require specialized handling, storage, and administration capabilities that are available primarily in hospital settings.
Based on the patient population, the hemophilia B gene therapy market is classified into severe hemophilia cases, moderate severity patients, and pediatric treatment groups. The severe hemophilia segment holds the largest market share due to urgent medical needs and higher treatment priority for patients with life-threatening bleeding episodes.
North America leads the global hemophilia B gene therapy market due to advanced healthcare infrastructure, significant research funding, and established frameworks for genetic medicine. The region is home to leading pharmaceutical companies, top research institutions, and favorable reimbursement policies. Strong Intellectual Property protection and venture capital investment support innovation and development. There are specialized treatment centers and experienced medical professionals to drive clinical development and patient access to new therapies. Regulatory support from agencies like the FDA also accelerates approval timelines and encourages early adoption of novel gene therapies.
Key Market Players
Leading companies operating in the global hemophilia B gene therapy market include:
- Freeline Therapeutics
- CSL Behring
- Alnylam Pharmaceuticals
- Dimension Therapeutics
- Bayer AG
- Pfizer Inc.
- Sangamo Therapeutics
- Roche Holding AG
- Spark Therapeutics
- Genethon
- uniQure N.V.
- REGENXBIO Inc.
- BioMarin Pharmaceutical Inc.
- Novo Nordisk A/S
- Shire plc (now part of Takeda Pharmaceutical Company)
Recent Developments
- In January 2025, CSL Behring announced positive results from Phase III clinical trials of its next-generation hemophilia B gene therapy, demonstrating sustained factor IX expression levels and improved bleeding control in patients with severe disease.
The global hemophilia B gene therapy market is segmented as follows:
By Patient Age Group
- Geriatric
- Adults
- Pediatric
By Disease Severity
- Severe Hemophilia B
- Moderate Hemophilia B
- Mild Hemophilia B
By Region
- North America
- The U.S.
- Europe
- UK
- France
- Germany
- Asia Pacific
- China
- Japan
- India
- Latin America
- Brazil
- The Middle East and Africa
About Us:
Zion Market Research is an obligated company. We create futuristic, cutting-edge, informative reports ranging from industry reports, the company reports to country reports. We provide our clients not only with market statistics unveiled by avowed private publishers and public organizations but also with vogue and newest industry reports along with pre-eminent and niche company profiles. Our database of market research reports comprises a wide variety of reports from cardinal industries. Our database is been updated constantly in order to fulfill our clients with prompt and direct online access to our database. Keeping in mind the client’s needs, we have included expert insights on global industries, products, and market trends in this database. Last but not the least, we make it our duty to ensure the success of clients connected to us—after all—if you do well, a little of the light shines on us.
Contact Us:
Zion Market Research
244 Fifth Avenue, Suite N202
New York, 10001, United States
Tel: +49-322 210 92714
USA/Canada Toll-Free No.1-855-465-4651
Email: sales@zionmarketresearch.com
Website: https://www.zionmarketresearch.com
Zion Market Research
Tel: +1 (302) 444-0166
USA/Canada Toll Free No.+1 (855) 465-4651
3rd Floor,
Mrunal Paradise, Opp Maharaja Hotel,
Pimple Gurav, Pune 411061,
Maharashtra, India
Phone No +91 7768 006 007, +91 7768 006 008
US OFFICE NO +1 (302) 444-0166
US/CAN TOLL FREE +1 (855) 465-4651
Email: sales@zionmarketresearch.com
We have secured system to process your transaction.
Our support available to help you 24 hours a day, five days a week.
Monday - Friday: 9AM - 6PM
Saturday - Sunday: Closed